��Ŀ����
�������ϣ�CaO��MgO�ڸ�������C�ѷ�Ӧ��������SiO2��Ӧ��������������һ�ֹ�ҵ��������Fe2O3������SiO2��Al2O3��CaO��MgO�����ʣ�����������������ȡ�����������廯�����������������£�
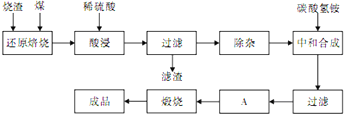
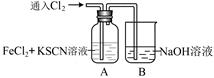
��1������ʱ��������Ҫ�к�������______��
��2��Ϊ�ⶨ����������Һ��Fe2+�ĺ�����ʵ�����г������Ը��������Һ���еζ���д���÷�Ӧ�����ӷ���ʽ��______��ʵ�����������Ը��������ҺŨ��Ϊ0.10mol/L����Һ��______�ζ�����ȡ20.00ml�������Ը��������Һ�ζ����յ�ʱ�����˱������Ը��������Һ12.04ml����Һ��c��Fe2+��=______��
��3�����ڿ����С������ʱ���������Һ��Fe2+�������½�����ԭ���ǣ�______�������ӷ���ʽ��ʾ����
��4�������±����ݣ�
�ڡ����ӡ������У�Ϊ��ȥFe3+��Al3+����Һ��pH���ֵӦС��______������Fe3+�Ѿ��������Լ���______����KSCN�⣩��
��5�����кͺϳɡ���Ŀ���ǽ���Һ��Fe2+ת��Ϊ̼��������������A�IJ�����______��

��1������ʱ��������Ҫ�к�������______��
��2��Ϊ�ⶨ����������Һ��Fe2+�ĺ�����ʵ�����г������Ը��������Һ���еζ���д���÷�Ӧ�����ӷ���ʽ��______��ʵ�����������Ը��������ҺŨ��Ϊ0.10mol/L����Һ��______�ζ�����ȡ20.00ml�������Ը��������Һ�ζ����յ�ʱ�����˱������Ը��������Һ12.04ml����Һ��c��Fe2+��=______��
��3�����ڿ����С������ʱ���������Һ��Fe2+�������½�����ԭ���ǣ�______�������ӷ���ʽ��ʾ����
��4�������±����ݣ�
| �������� | Al��OH��3 | Fe��OH��3 | Fe��OH��2 |
| ��ʼ������pH | 3.10 | 2.01 | 7.11 |
| ��ȫ������pH | 4.77 | 3.68 | 9.61 |
��5�����кͺϳɡ���Ŀ���ǽ���Һ��Fe2+ת��Ϊ̼��������������A�IJ�����______��
��1��������������һ�ֹ�ҵ��������Fe2O3������SiO2��Al2O3��CaO��MgO�����ʣ����������̷���������̼��ԭ��Ͷ������跴Ӧ���ɹ��һ����̼��������ȼ�ջ����ɶ����������ɵ���Ⱦ������Ϊһ����̼�Ͷ�������
�ʴ�Ϊ��CO��SO2��
��2�����ݸ��������Һ������������Ϊ�����ӣ���������ԭΪ�����ӣ����ݵ�� �غ㣬�����غ㣬ԭ���غ���ƽ��д���ӷ���ʽΪ��MnO4-+5Fe2++8H+�TMn2++5Fe3++4H2O����Һ��������Ҫ�����Եζ���ȡ�ã��������ӷ���ʽ����õ���Һ�������������ʵ�����
MnO4-+5Fe2++8H+�TMn2++5Fe3++4H2O��
1 5
0.10mol/L��0.012.04l n��Fe2+��
n��Fe2+��=0.00602mol
�õ��������ӵ�Ũ��Ϊ
=0.0301mol/L��
�ʴ�Ϊ����ʽ��0.0301mol/L��
��3���ڿ����С������ʱ���������Һ��Fe2+�������½���ԭ��������������������Һ�б���������Ϊ�����ӣ���Ӧ�����ӷ���ʽΪ��4Fe2++O2+4H+�T4Fe3++2H2O��
�ʴ�Ϊ��4Fe2++O2+4H+�T4Fe3++2H2O��
��4����ȥFe3+��Al3+������ͼ���г�������PH��������ҺPHС��7.11ʱ���������Ӳ������������Ӻ���������ȫ���������������ӳ�KSCN�⣬���Լ��뱽�ӳ�����ɫ֤�������ӵĴ��ڣ�
�ʴ�Ϊ��7.11�����ӣ�
��5������Һ��Fe2+ת��Ϊ̼��������������ֹ�������ʣ���Ҫ�Գ�������ϴ�Ӹ��
�ʴ�Ϊ��ϴ�ӻ�ϴ�ӡ����
�ʴ�Ϊ��CO��SO2��
��2�����ݸ��������Һ������������Ϊ�����ӣ���������ԭΪ�����ӣ����ݵ�� �غ㣬�����غ㣬ԭ���غ���ƽ��д���ӷ���ʽΪ��MnO4-+5Fe2++8H+�TMn2++5Fe3++4H2O����Һ��������Ҫ�����Եζ���ȡ�ã��������ӷ���ʽ����õ���Һ�������������ʵ�����
MnO4-+5Fe2++8H+�TMn2++5Fe3++4H2O��
1 5
0.10mol/L��0.012.04l n��Fe2+��
n��Fe2+��=0.00602mol
�õ��������ӵ�Ũ��Ϊ
| 0.00602mol |
| 0.02L |
�ʴ�Ϊ����ʽ��0.0301mol/L��
��3���ڿ����С������ʱ���������Һ��Fe2+�������½���ԭ��������������������Һ�б���������Ϊ�����ӣ���Ӧ�����ӷ���ʽΪ��4Fe2++O2+4H+�T4Fe3++2H2O��
�ʴ�Ϊ��4Fe2++O2+4H+�T4Fe3++2H2O��
��4����ȥFe3+��Al3+������ͼ���г�������PH��������ҺPHС��7.11ʱ���������Ӳ������������Ӻ���������ȫ���������������ӳ�KSCN�⣬���Լ��뱽�ӳ�����ɫ֤�������ӵĴ��ڣ�
�ʴ�Ϊ��7.11�����ӣ�
��5������Һ��Fe2+ת��Ϊ̼��������������ֹ�������ʣ���Ҫ�Գ�������ϴ�Ӹ��
�ʴ�Ϊ��ϴ�ӻ�ϴ�ӡ����

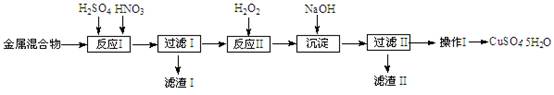
��ϰ��ϵ�д�
�����Ŀ
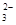 = 2I����S4O
= 2I����S4O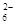
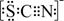 ��
��