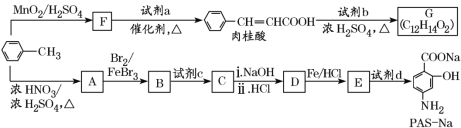
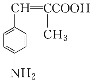
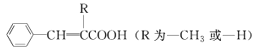ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ“―÷ΣΡ≥»ή“ΚXΩ…Ρή”…K+ΓΔMg2+ΓΔCu2+ΓΔAg+ΓΔBa2+ΓΔAl3+ΓΔFe2+ΓΔ![]() ΓΔ
ΓΔ![]() ΓΔ
ΓΔ![]() ΓΔI-ΓΔ
ΓΔI-ΓΔ![]() ΓΔ
ΓΔ![]() ÷–ΒΡ»τΗ…÷÷άκΉ”Ήι≥…ΓΘΡ≥Μ·―ß–Υ»Λ–ΓΉιΆ®Ιΐœ¬Ν– Β―ι»ΖΕ®ΝΥΤδΉι≥…ΓΘ
÷–ΒΡ»τΗ…÷÷άκΉ”Ήι≥…ΓΘΡ≥Μ·―ß–Υ»Λ–ΓΉιΆ®Ιΐœ¬Ν– Β―ι»ΖΕ®ΝΥΤδΉι≥…ΓΘ
(1)ΗυΨίœ¬Ν– Β―ι≤Ϋ÷ηΚΆœ÷œσΘ§ΆΤΕœ Β―ιΫα¬έΘΚ
Β―ι≤Ϋ÷η”κ Β―ιœ÷œσ | Β―ιΫα¬έ |
Δώ.Ιέ≤λ»ή“ΚΘΚΈό…ΪΆΗΟς | ΔΌ‘≠»ή“Κ÷–“ΜΕ®≤ΜΚ§ΒΡάκΉ” «____ΓΘ |
Δρ.»Γ ΝΩΗΟ»ή“ΚΘ§Φ”»κΙΐΝΩΒΡœθΥαΘ§”–ΤχΧε…ζ≥…Θ§≤ΔΒΟΒΫΈό…Ϊ»ή“Κ | ΔΎ‘≠»ή“Κ÷–“ΜΕ®≤ΜΚ§ΒΡάκΉ” «_____Θ§“ΜΕ®Κ§”–ΒΡάκΉ” «____ΓΘ |
Δσ.‘ΎΔρΥυΒΟ»ή“Κ÷–‘ΌΦ”»κΙΐΝΩΒΡΧΦΥα«βοß»ή“ΚΘ§”–ΤχΧε…ζ≥…Θ§Ά§ ±Έω≥ωΑΉ…Ϊ≥ΝΒμA | Δέ‘≠»ή“Κ÷–ΜΙ“ΜΕ®Κ§”–ΒΡάκΉ” «____Θ§…ζ≥…≥ΝΒμAΒΡάκΉ”ΖΫ≥Χ ΫΈΣ____ΓΘ |
Δτ.‘ΎΔσΥυΒΟ»ή“Κ÷–‘Ό÷πΒΈΦ”»κ«β―θΜ·±Β»ή“Κ÷ΝΙΐΝΩΘ§Φ”»»“≤”–ΤχΧε…ζ≥…Θ§Ά§ ±Έω≥ωΑΉ…Ϊ≥ΝΒμB | ΔήΑΉ…Ϊ≥ΝΒμB÷–“ΜΕ®Κ§”–___Θ§Ω…ΡήΚ§”–____ΓΘ |
(2)…œ ω Β―ι≤Ϋ÷ηΔτ÷–ΩΣ ΦΫΉΕΈ“ΜΕ®ΖΔ…ζΒΡάκΉ”ΖΫ≥Χ Ϋ «______ΓΘ
(3)ΗΟΜ·―ß–Υ»Λ–ΓΉιΒΡΆ§―ßΈΣΝΥΫχ“Μ≤Ϋ»ΖΕ®BΒΡ≥…Ζ÷Θ§»Γ“ΜΕ®ΝΩΨ≠œ¥Β”ΚσΒΡB”κY»ή“ΚΖ¥”ΠΘ§ΑΉ…ΪΙΧΧεΒΡΈο÷ ΒΡΝΩ”κY»ή“ΚΧεΜΐ÷°ΦδΒΡΙΊœΒ»γΆΦΥυ ΨΓΘ

YΩ…ΡήΈΣ(ΧνΜ·―ß Ϋ)____Θ§BΒΡΉι≥…ΈΣ______ΓΘ
ΓΨ¥πΑΗΓΩCu2+ΓΔFe2+ΓΔ![]() I-ΓΔ
I-ΓΔ![]() ΓΔMg2+ΓΔAg+ΓΔBa2+ΓΔAl3+
ΓΔMg2+ΓΔAg+ΓΔBa2+ΓΔAl3+ ![]() ΓΔK+
ΓΔK+ ![]() Al3+ΘΪ3
Al3+ΘΪ3![]() ===Al(OH)3ΓΐΘΪ3CO2Γϋ BaCO3 BaSO4 Ba2+ΘΪ2OH-ΘΪ2
===Al(OH)3ΓΐΘΪ3CO2Γϋ BaCO3 BaSO4 Ba2+ΘΪ2OH-ΘΪ2![]() ===BaCO3ΓΐΘΪ
===BaCO3ΓΐΘΪ![]() ΘΪ2H2O HCl(ΜρHNO3) BaSO4ΚΆBaCO3Θ§«“
ΘΪ2H2O HCl(ΜρHNO3) BaSO4ΚΆBaCO3Θ§«“![]() ΘΫ
ΘΫ![]()
ΓΨΫβΈωΓΩ
IΘ°Ιέ≤λ»ή“ΚΘΚΈό…ΪΆΗΟςΘ§“ΜΕ®≤Μ¥φ‘Ύ”–…ΪάκΉ”ΘΚCu2ΘΪΓΔ![]() ΓΔFe2ΘΪΘΜ
ΓΔFe2ΘΪΘΜ
ΔρΘ°»Γ ΝΩΗΟ»ή“ΚΘ§Φ”»κΙΐΝΩΒΡœθΥαΘ§”–ΤχΧε…ζ≥…Θ§ΗΟΤχΧεΈΣΕΰ―θΜ·ΧΦΘ§»ή“Κ÷–“ΜΕ®¥φ‘Ύ![]() Θ§“ΜΕ®≤Μ¥φ‘Ύ”κCO32Θ≠άκΉ”Ζ¥”ΠΒΡMg2ΘΪΓΔAgΘΪΓΔBa2ΘΪΓΔAl3ΘΪάκΉ”ΘΜΒΟΒΫΈό…Ϊ»ή“ΚΘ§“ΜΕ®≤Μ¥φ‘ΎIΘ≠ΓΔ
Θ§“ΜΕ®≤Μ¥φ‘Ύ”κCO32Θ≠άκΉ”Ζ¥”ΠΒΡMg2ΘΪΓΔAgΘΪΓΔBa2ΘΪΓΔAl3ΘΪάκΉ”ΘΜΒΟΒΫΈό…Ϊ»ή“ΚΘ§“ΜΕ®≤Μ¥φ‘ΎIΘ≠ΓΔ![]() Θ§‘ΌΗυΨί»ή“Κ≥ Βγ÷––‘≈–Εœ‘≠»ή“Κ÷–“ΜΕ®¥φ‘ΎΈ®“Μ―τάκΉ”KΘΪΘΜ
Θ§‘ΌΗυΨί»ή“Κ≥ Βγ÷––‘≈–Εœ‘≠»ή“Κ÷–“ΜΕ®¥φ‘ΎΈ®“Μ―τάκΉ”KΘΪΘΜ
ΔσΘ°‘ΎΔρΥυΒΟ»ή“Κ÷–‘ΌΦ”»κΙΐΝΩNH4HCO3»ή“ΚΘ§”–ΤχΧε…ζ≥…Θ§Ά§ ±Έω≥ωΑΉ…Ϊ≥ΝΒμAΘ§ΥΒΟς»ή“Κ÷–“ΜΕ®¥φ‘Ύ¬ΝάκΉ”Θ§‘≠»ή“Κ÷–“ΜΕ®¥φ‘Ύ![]() ΘΜ
ΘΜ
IVΘ°‘ΎΔσΥυΒΟ»ή“Κ÷–Κ§”–ΙΐΝΩΒΡΧΦΥα«βοßΘ§Φ”»κΙΐΝΩBa(OH)2»ή“Κ÷ΝΙΐΝΩΘ§Φ”»»Μα”–Α±Τχ…ζ≥…Θ§ΑΉ…Ϊ≥ΝΒμΩ…ΡήΈΣΧΦΥα±ΒΜρΧΦΥα±ΒΚΆΝρΥα±ΒΒΡΜλΚœΈοΓΘ
(1)IΘ°Ιέ≤λ»ή“ΚΘΚΈό…ΪΆΗΟςΘ§“ΜΕ®≤Μ¥φ‘Ύ”–…ΪάκΉ”ΘΚCu2ΘΪΓΔMnO4Θ≠ΓΔFe2ΘΪΘΜΙ ¥πΑΗΈΣΘΚCu2ΘΪΓΔMnO4Θ≠ΓΔFe2ΘΪΘΜ
ΔρΘ°»Γ ΝΩΗΟ»ή“ΚΘ§Φ”»κΙΐΝΩΒΡœθΥαΘ§”–ΤχΧε…ζ≥…Θ§ΗΟΤχΧεΈΣΕΰ―θΜ·ΧΦΘ§»ή“Κ÷–“ΜΕ®¥φ‘Ύ![]() Θ§“ΜΕ®≤Μ¥φ‘Ύ”κ
Θ§“ΜΕ®≤Μ¥φ‘Ύ”κ![]() άκΉ”Ζ¥”ΠΒΡMg2ΘΪΓΔAgΘΪΓΔBa2ΘΪΓΔAl3ΘΪάκΉ”ΘΜΒΟΒΫΈό…Ϊ»ή“ΚΘ§“ΜΕ®≤Μ¥φ‘ΎIΘ≠ΓΔ
άκΉ”Ζ¥”ΠΒΡMg2ΘΪΓΔAgΘΪΓΔBa2ΘΪΓΔAl3ΘΪάκΉ”ΘΜΒΟΒΫΈό…Ϊ»ή“ΚΘ§“ΜΕ®≤Μ¥φ‘ΎIΘ≠ΓΔ![]() Θ§‘ΌΗυΨί»ή“Κ≥ Βγ÷––‘≈–Εœ‘≠»ή“Κ÷–“ΜΕ®¥φ‘ΎΈ®“Μ―τάκΉ”KΘΪΘΜΙ ¥πΑΗΈΣΘΚI-ΓΔ
Θ§‘ΌΗυΨί»ή“Κ≥ Βγ÷––‘≈–Εœ‘≠»ή“Κ÷–“ΜΕ®¥φ‘ΎΈ®“Μ―τάκΉ”KΘΪΘΜΙ ¥πΑΗΈΣΘΚI-ΓΔ![]() ΓΔMg2+ΓΔAg+ΓΔBa2+ΓΔAl3+ΘΜ
ΓΔMg2+ΓΔAg+ΓΔBa2+ΓΔAl3+ΘΜ
ΔσΘ°‘ΎΔρΥυΒΟ»ή“Κ÷–‘ΌΦ”»κΙΐΝΩNH4HCO3»ή“ΚΘ§”–ΤχΧε…ζ≥…Θ§Ά§ ±Έω≥ωΑΉ…Ϊ≥ΝΒμAΘ§ΥΒΟς»ή“Κ÷–“ΜΕ®¥φ‘Ύ¬ΝάκΉ”Θ§‘≠»ή“Κ÷–“ΜΕ®¥φ‘Ύ![]() Θ§Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣAl3ΘΪ+3
Θ§Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣAl3ΘΪ+3![]() =Al(OH)3Γΐ+3CO2ΓϋΘ§Ι ¥πΑΗΈΣΘΚ
=Al(OH)3Γΐ+3CO2ΓϋΘ§Ι ¥πΑΗΈΣΘΚ![]() ΘΜAl3+ΘΪ3
ΘΜAl3+ΘΪ3![]() =Al(OH)3ΓΐΘΪ3CO2ΓϋΘΜ
=Al(OH)3ΓΐΘΪ3CO2ΓϋΘΜ
IVΘ°‘ΎΔσΥυΒΟ»ή“Κ÷–Κ§”–ΙΐΝΩΒΡΧΦΥα«βοßΘ§Φ”»κΙΐΝΩBa(OH)2»ή“Κ÷ΝΙΐΝΩΘ§Φ”»»Μα”–Α±Τχ…ζ≥…Θ§ΑΉ…Ϊ≥ΝΒμΩ…ΡήΈΣΧΦΥα±ΒΜρΧΦΥα±ΒΚΆΝρΥα±ΒΒΡΜλΚœΈοΘ§Ι ¥πΑΗΈΣΘΚBaCO3ΘΜBaSO4ΘΜ
(2) Β―ιΔτ÷–ΩΣ ΦΫΉΕΈΖΔ…ζΖ¥”ΠΈΣΙΐΝΩΒΡΧΦΥα«βοß”κ«β―θΜ·±ΒΖ¥”ΠΘ§Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣΘΚ2HCO3Θ≠+Ba2ΘΪ+2OH=BaCO3Γΐ+2H2O+![]() Θ§Ι ¥πΑΗΈΣΘΚ2HCO3Θ≠+Ba2ΘΪ+2OH=BaCO3Γΐ+2H2O+
Θ§Ι ¥πΑΗΈΣΘΚ2HCO3Θ≠+Ba2ΘΪ+2OH=BaCO3Γΐ+2H2O+![]() ΘΜ
ΘΜ
(3)≥ΝΒμB≤ΩΖ÷»ή“ΚYΥαΘ§YΩ…ΡήΈΣœθΥαΜρ―ΈΥαΘ§‘ρB÷–“ΜΕ®Κ§”–BaCO3ΓΔBaSO4Θ§”…ΆΦœσΩ…÷Σ![]() =
=![]() Θ§Ι ¥πΑΗΈΣΘΚHClΜρHNO3ΘΜBaCO3ΓΔBaSO4«“
Θ§Ι ¥πΑΗΈΣΘΚHClΜρHNO3ΘΜBaCO3ΓΔBaSO4«“![]() =
=![]() ΓΘ
ΓΘ

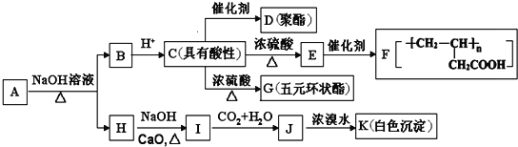
ΓΨΧβΡΩΓΩΝρΥα–Ω «÷Τ‘λ–Ω±ΒΑΉΚΆ–Ω―ΈΒΡ÷ς“Σ‘≠ΝœΘ§“≤Ω…”ΟΉςΡΨ≤ΡΒΡΖάΗ·ΦΝΒ»ΓΘ”Ο―θΜ·–Ω―Χ≥Ψ(÷ς“Σ≥…Ζ÷ΈΣZnOΘ§ΜΙΚ§”–…ΌΝΩPbOΓΔCuOΓΔFe2 O3ΓΔFeOΒ»)…ζ≤ζZnSO4ΓΛ7H2OΒΡΝς≥Χ»γœ¬ΘΚ

”–ΙΊΫπ τάκΉ”[c(Mn+)= 0.l mol/L]–Έ≥…«β―θΜ·Έο≥ΝΒμΒΡpHΖΕΈß»γœ¬±μΘΚ
Ϋπ τάκ | Fe3+ | Fe2+ | Zn2+ | Cu2+ |
ΩΣ Φ≥ΝΒμΒΡpH | 1.5 | 6.3 | 6.2 | 4.7 |
≥ΝΒμΆξ»ΪΒΡpH | 2.8 | 8.3 | 8.2 | 6.7 |
(1)ΓΑΥαΫΰΓ± ±”ΟΒΡœΓΥα «____ΘΜ¬Υ‘ϋ1÷ς“Σ≥…Ζ÷ «____ΓΘ
(2)ΓΑ―θΜ·Γ± ±ΒΡάκΉ”ΖΫ≥Χ ΫΈΣ_________ΘΜΦ”»κZnO≥ΐ‘” ±»ή“ΚΒΡpHΩΊ÷ΤΖΕΈß «____ΓΪ5.0ΓΘ
(3)¬Υ‘ϋ3Κ§”––ΩΚΆ____ΘΜ¬Υ“ΚΒΟΒΫZnSO4ΓΛ7H2OΒΡ≤ΌΉς «____ΓΔœ¥Β”ΓΔΗ…‘οΓΘ
(4)»Γ14.35gZnSO4ΓΛ7H2OΦ”»»÷Ν≤ΜΆ§Έ¬Ε»Θ§ Θ”ύΙΧΧεΒΡ÷ ΝΩ»γœ¬±μ
Έ¬Ε»Θ·Γφ | 100 | 250 | 680 | 930 |
÷ ΝΩ/g | 8. 95 | 8. 05 | 6. 72 | 4.05 |
‘ρ680Γφ ± Θ”ύΙΧΧεΒΡΜ·―ß ΫΈΣ________(Χν–ρΚ≈)ΓΘ
AΘ°ZnO BΘ°Zn3O(SO4