��Ŀ����
[��ѧѡ�ޡ���3�����ʽṹ������]��15�֣�
���ڱ�ǰ�����ڵ�Ԫ��a��b��c��d��e��ԭ��������������A�ĺ����������������Ӳ�����ͬ��b�ļ۵��Ӳ��е�δ�ɶԵ�����3����c������������Ϊ���ڲ��������3����d��cͬ���壬e�������ֻ��1�����ӣ����������18�����ӡ��ش��������⣺
��1��b��c��d�е�һ������������ ����Ԫ�ط��ţ���e�ļ۲���ӹ��ʾ��ͼΪ ��
��2��a������Ԫ���γɵĶ�Ԫ���ۻ������У����ӳ������Σ��÷��ӵ�����ԭ�ӵ��ӻ���ʽΪ �������мȺ��м��Թ��ۼ����ֺ��зǼ��Թ��ۼ��Ļ������� ���ѧʽ��д���֣���
��3����ЩԪ���γɵĺ������У����ӵ�����ԭ�ӵļ۲���Ӷ���Ϊ3������ ������������ṹ������ �����ѧʽ��
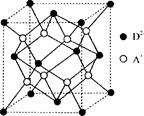
��4��c��e�γɵ�һ�����ӻ�����ľ���ṹ��ͼ1����e���ӵĵ��Ϊ ��
��5����5��Ԫ���γɵ�һ��1:1�����ӻ������У������ӳ�������ṹ�������ӳ����������İ�����ṹ����ͼ2��ʾ�����û�������������Ϊ ���������д��ڵĻ�ѧ�������� ���û��������ʱ����ʧȥ������� ���ж������� ��
��1��N 
��2��sp3 H2O2��N2H4
��3��HNO2��HNO3 H2SO3
��4��+1
��5��SO42�� ���ۼ�����λ�� H2O H2O��Cu2������λ����NH3��Cu2������
���������������������֪�����ڱ�ǰ�����ڵ�Ԫ��a��b��c��d��e��ԭ��������������a�ĺ����������������Ӳ�����ͬ����aΪ��Ԫ�أ�b�ļ۵��Ӳ��е�δ�ɶԵ�����3������bΪ��Ԫ�أ�c������������Ϊ���ڲ��������3������cΪ��Ԫ�أ�d��cͬ���壬��dΪ��Ԫ�أ�e�������ֻ��1�����ӣ����������18�����ӣ���eΪͭԪ�ء���1��ͬ�����������ҵ�һ�����ܳʵ������ƣ�����Ԫ��ԭ�ӵĹ����ȫ����ȫ�ա������״̬ʱ�����ȶ���ͬ�������ϵ��µ�һ��������С����ԭ��2p���Ϊ�����״̬�����ȶ�����N��O��S�е�һ������������N��eΪͭԪ�أ��۲���ӹ��ʾ��ͼΪ ����2��aΪ��Ԫ�أ�������Ԫ���γɵĶ�Ԫ���ۻ������У����ӳ������Σ��÷���Ϊ�����ӣ�����ԭ�ӵ��ӻ���ʽΪsp3�������мȺ��м��Թ��ۼ����ֺ��зǼ��Թ��ۼ��Ļ�������H2O2��N2H4��C2H6�ȡ���3����ЩԪ���γɵĺ������У����ӵ�����ԭ�ӵļ۲���Ӷ���Ϊ3������HNO2��HNO3������������ṹ������H2SO3����4������O��Cu�γɵ����ӻ�����ľ����ṹ�жϣ��û�����Ļ�ѧʽΪCu2O����e���ӵĵ��Ϊ+1����5�����������Ϣ֪����5��Ԫ���γɵ�һ��1:1�����ӻ������У������ӳ�������ṹ��Ϊ������������ӳ����������İ�����ṹ���ͼ2֪���û�����Ļ�ѧʽΪ[Cu(NH3)4(H2O)2]SO4����û�������������ΪSO42�����������д��ڵĻ�ѧ�������й��ۼ�����λ�����û��������ʱ����ʧȥ�������H2O���ж�������H2O��Cu2������λ����NH3��Cu2��������
����2��aΪ��Ԫ�أ�������Ԫ���γɵĶ�Ԫ���ۻ������У����ӳ������Σ��÷���Ϊ�����ӣ�����ԭ�ӵ��ӻ���ʽΪsp3�������мȺ��м��Թ��ۼ����ֺ��зǼ��Թ��ۼ��Ļ�������H2O2��N2H4��C2H6�ȡ���3����ЩԪ���γɵĺ������У����ӵ�����ԭ�ӵļ۲���Ӷ���Ϊ3������HNO2��HNO3������������ṹ������H2SO3����4������O��Cu�γɵ����ӻ�����ľ����ṹ�жϣ��û�����Ļ�ѧʽΪCu2O����e���ӵĵ��Ϊ+1����5�����������Ϣ֪����5��Ԫ���γɵ�һ��1:1�����ӻ������У������ӳ�������ṹ��Ϊ������������ӳ����������İ�����ṹ���ͼ2֪���û�����Ļ�ѧʽΪ[Cu(NH3)4(H2O)2]SO4����û�������������ΪSO42�����������д��ڵĻ�ѧ�������й��ۼ�����λ�����û��������ʱ����ʧȥ�������H2O���ж�������H2O��Cu2������λ����NH3��Cu2��������
���㣺�������ʽṹ�����ʣ��漰Ԫ���ƶϡ�Ԫ�������ɣ���������Ų����ɣ����ۼ�����ӽṹ�����ʣ�����ṹ���֪ʶ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�(11��)A��B��C��D��E��F���ֶ���������Ԫ�أ����ǵ�ԭ��������������A�ж��ֺ��أ�����һ��û�����ӣ�BԪ�ص�����������Ӧˮ���������⻯���������Σ�D��Aͬ���壬����Eͬ���ڣ�EԪ��ԭ�ӵ��������������������������3/4��A��B��D��E������Ԫ�أ�ÿһ����CԪ�ض����γ�ԭ�Ӹ����Ȳ�ͬ�Ķ��ֻ������ش��������⣺
��1�� F��Ԫ�����ڱ��е�λ����_______________;�õ���ʽ��ʾA��F����Ԫ����ɵĻ������
�γɹ���______________________________________________��
��֪�±��е��������ƻ�1 mol�����еĻ�ѧ�������յ�������kJ����Ԫ��A�ĵ�����Ԫ��F�ĵ�����һ�������·�Ӧ����2mol����ʱ�ͷŵ�����Ϊ____________kJ��
| ��ѧ�� | Cl2 | Br2 | I2 | HCl | HBr | HI | H2 |
| ������kJ�� | 243 | 193 | 151 | 432 | 366 | 298 | 436 |
��������֤E��F����Ԫ�طǽ���ǿ�����ǣ���д��ĸ��
A���Ƚ�������Ԫ�صij������ʵķе�
B���Ƚ�������Ԫ�صĵ������������ϵ�����
C���Ƚ�������Ԫ�ص����������ˮ���������
��3�� A��C��E����֮����γɼס��������������Ǿ�Ϊ��һ����λ����ɵ�˫ԭ�������ӣ��Ҽ���18�����ӣ�����10�����ӣ�������ҷ�Ӧ�����ӷ���ʽΪ
(15��)
A��B��D��E��FΪ������Ԫ�أ��ǽ���Ԫ��A����������������������ͬ��B����������������������������2����B ��D�г��ȼ������������ۻ�����BD2��E����D2��������ͬ�ĵ�������A��F��ȼ�գ���������ˮ�õ�һ��ǿ�ᡣ�ش��������⣺
��1��A�����ڱ��е�λ���� ��д��һ�ֹ�ҵ�Ʊ�����F�����ӷ���ʽ ��
��2��B��D��E��ɵ�һ�����У�E����������Ϊ43%��������Ϊ ����ˮ��Һ��F���ʷ�Ӧ�Ļ�ѧ����ʽΪ ���ڲ����м�������KI����Ӧ�����CC14������ ������ ɫ��
��3������ЩԪ����ɵ����ʣ�����ɺͽṹ��Ϣ���±���
| ���� | ��ɺͽṹ��Ϣ |
| a | ����A�Ķ�Ԫ���ӻ����� |
| b | ���зǼ��Թ��ۼ��Ķ�Ԫ���ӻ������ԭ����֮��Ϊ1:1 |
| c | ��ѧ���ΪBDF2 |
| d | ֻ����һ�������������ҿɵ���ĵ��ʾ��� |
d�ľ��������� ��
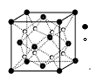
��4����A��B��DԪ����ɵ����ֶ�Ԫ�������γ�һ������Դ���ʡ�һ�ֻ��������ͨ�� �����ɾ��п�ǻ�Ĺ��壻��һ�ֻ�����(��������Ҫ�ɷ�)���ӽ���ÿ�ǻ������ӵĿռ�ṹΪ ��
��13�֣�������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ������ͼ��ʾ������T��������������������������ȣ���ش��������⣺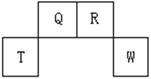
(1)W�����ڱ��е�λ���� �� Q��R��T����Ԫ��ԭ�ӵİ뾶�Ӵ�С����˳�� ����Ԫ�ط��ű�ʾ����Q�����������ĵ���ʽ ��R��̬�⻯����ӵĽṹʽΪ ��
(2)Ԫ�ص�ԭ�ӵõ���������Q W���ǿ�ڡ������ڡ�����
(3)ԭ��������R��8��Ԫ���γɵ�һ�ֳ�����̬�⻯��ķе� ����ߡ��͡�����R�ĵij�����̬�⻯�
(4)T��Q��R��W�ĵ����У���̬ʱ����ԭ�Ӿ������ �������ƣ���
(5)����8��Ԫ�ص����ʡ��������±����У��������ڶ����ڣ���ָ��RԪ�����±��еĶ�Ӧ��� ����Tͬ����������������ˮ���������ǿ��Ԫ�����±��еĶ�Ӧ��� ��
| | �� | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶��10-10m�� | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.6 | 0.75 | 0.82 |
| �����ͻ��ϼ� | | +2 | +1 | +5 | +7 | +1 | +5 | +3 |
| -2 | | | -3 | -1 | | -3 | |