��Ŀ����
18����ҵ��һ���Ʊ��Ȼ�����������صĹ���������ͼ��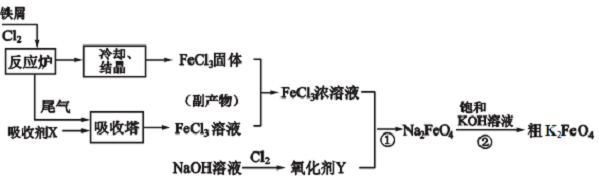
��1���������е����ռ�X��FeCl2���Ӹ�����FeCl3��Һ�л��FeCl3•6H2O�IJ����Ǽ����������ᣨ��ͨ��HCl��������Ũ��������ȴ�ᾧ��
��2����FeCl3��Һ���������ʴӡˢ��·�����õķ�Һ�к�FeCl3��FeCl2��CuCl2���û�ѧ�������Ի��շ�Һ��ͭ���ϲ����˺��ʣ��Һ�������Ϊ�������������е����ռ�X�����ڴ˹����У��Ⱥ��������ʷֱ���Fe����м����HCl�����ᣩ��
��3�����������·�Ӧ�ٵ����ӷ���ʽΪ2Fe3++3ClO-+10OH-�T2FeO42-+3Cl-+5H2O��
��4�����̢ڽ������Һ�����Сʱ�����ã����˻�ôֲ�Ʒ���÷�Ӧ�Ļ�ѧ����ʽΪ2KOH+Na2FeO4=K2FeO4+2NaOH������ݸ��ֽⷴӦԭ��������Ӧ������ԭ��K2FeO4�ܽ��С���������壬�ٽ���Ӧ���У�
K2FeO4 ��ˮ��Һ��������Ӧ��4FeO42-+10H2O�T4Fe��OH��3+8OH-+3O2�������ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��b������ţ���
a��H2O b��ϡKOH��Һ�������
c��NH4Cl��Һ������� d��Fe��NO3��3��Һ���������
���� ����������Ӧ���ɺ��ص��̣��������������Ȼ�������Һ���գ������Ȼ�����Һ������ɫ�Ȼ������Ȼ�����Һ���գ�����Ũ���Ȼ�����Һ��Ȼ���Ȼ���������������������Һ��Ӧ���ɵĴ������������ɸ������ƣ����ͨ����Ӧ2KOH+Na2FeO4=K2FeO4+2NaOH���Ƶø�����أ��ɴ˷������
��1���ӷ�Ӧ¯���ų���β���Ƿ�Ӧʣ���Cl2�������ռ�X��Ӧ����FeCl3��Һ������X���Ȼ�������Һ����ֹ�����ӵ�ˮ�⣬����Ҫ�������
��2�����ȼ�����������۽�������ת��Ϊ�������ӣ�ͭ����ת��Ϊ����ͭ��Ȼ����������������ȥ���������ۣ������˵õ�����ͭ��
��3������������������Ӧ����NaClO������������FeCl3��Ӧ����FeO42-��
��4�����̢ڽ������Һ�����Сʱ�����ã����˻�ôֲ�Ʒ���÷�Ӧ�Ļ�ѧ����ʽΪ2KOH+Na2FeO4 =K2FeO4+2NaOH���������ط�Ӧԭ��������Ӧ�ܷ�����ԭ��K2FeO4���ܽ�ȱ�Na2FeO4С����Һ��K+��FeO42-��Ũ�ȱȽϴ�K2FeO4 ��ˮ��Һ��������Ӧ��4FeO42-+10H2O�T4Fe��OH��3+8OH-+3O2���������ü�Һ��ϴ�ӣ�����ˮ�⣬�������µ�����������ϡKOH��Һ��������ӷ���
��� �⣺��1��ͨ����������ͼ��֪���ӷ�Ӧ¯���ų���β���Ƿ�Ӧʣ���Cl2�������ռ�X���������з�Ӧ����FeCl3��Һ�������ռ�XӦ��FeCl2��Һ����ֹ�����ӵ�ˮ�⣬����Ҫ���ᣬ���������µ����ʣ����Լ����ᣬ�ʴ�Ϊ��FeCl2�������������ᣨ��ͨ��HCl����
��2�����ȼ�����������۽�������ת��Ϊ�������ӣ�ͭ����ת��Ϊ����ͭ��Ȼ����������������ȥ���������ۣ������˵õ�����ͭ���ʴ�Ϊ��Fe����м����HCl�����ᣩ��
��3��NaClO��FeCl3��Ӧ����FeO42-���������Ʊ���ԭΪ�����ӣ���Ӧ�����ӷ���ʽΪ��2Fe3++3ClO-+10OH-�T2FeO42-+3Cl-+5H2O��
�ʴ�Ϊ��2Fe3++3ClO-+10OH-�T2FeO42-+3Cl-+5H2O��
��4�����̢ڽ������Һ�����Сʱ�����ã����˻�ôֲ�Ʒ���÷�Ӧ�Ļ�ѧ����ʽΪ2KOH+Na2FeO4 =K2FeO4+2NaOH���������ط�Ӧԭ��������Ӧ�ܷ�����ԭ��K2FeO4���ܽ�ȱ�Na2FeO4С����Һ��K+��FeO42-��Ũ�ȱȽϴ�K2FeO4 ��ˮ��Һ��������Ӧ��4FeO42-+10H2O�T4Fe��OH��3+8OH-+3O2���������ü�Һ��ϴ�ӣ�����ˮ�⣬�������µ�����������ϡKOH��Һ��������ӷ����ʴ�Ϊ��K2FeO4�ܽ��С���������壬�ٽ���Ӧ���У�b��
���� ���⿼�������ʵ��Ʊ����̵�����Ӧ�á�ʵ������������������ʵķ���Ӧ�ã���Ŀ�漰��֪ʶ��϶࣬�����ڿ���ѧ����ʵ�������ͶԻ���֪ʶ���ۺ�Ӧ���������������ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

 ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�
| A�� | ��װ�ü����Ҵ���Ũ����Ϊԭ������ϩ | |
| B�� | ��װ��������ijЩʵ��β���еĶ������� | |
| C�� | ��װ�ñ�����Cl2��KI��Һ��Ӧ���ɵĵ� | |
| D�� | ��װ�ö�����NH4Cl������Һ��ȡNH4Cl���� |
| ѡ�� | ��ʵ | ���� |
| A | ���ȵĴ�����Һϴȥ���� | Na2CO3����Ϊ��֬�ֽ�Ĵ��� |
| B | ��������������ϡ���� | �������γ���������Ĥ���б������� |
| C | ������Ϊ��ױƷ�еı�ʪ�� | ��������ˮ�γ���� |
| D | ������ʴˮ������Ʒ | HF����ǿ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��ά�ؿ���ˮ�����������ǣ������ά�����ڻ���Ӫ������ | |
| B�� | ���飨C5H12��������ͬ���칹�� | |
| C�� | �����屽���Ҵ�������ˮ���� | |
| D�� | �Ҵ�����ֱ������������ |
| A�� | SO3��NO3-��BF3����ƽ�������� | B�� | P4��CS2��PH3���ǷǼ��Է��� | ||
| C�� | ���ʯ��ʯī��SiC����ԭ�Ӿ��� | D�� | ��һ�����ܣ�N��O��C |
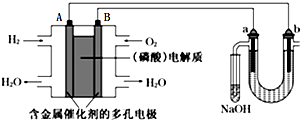
| A�� | ��ع���ʱ��B����ӦʽΪO2+2H2O+4e-=4OH- | |
| B�� | ���ʱ����������·���ǣ�A�������·��b������Һ��a����B�� | |
| C�� | NaOH��Һ������պ�������Һ������Ư��ˮ | |
| D�� | �����������2.24L����״����H2ʱ��a����ΧҲ�����2.24L����״�������� |
| A�� | ����Һ�������ӵ�Ũ�ȣ�c��H+��=1��10-11 mol•L-1 | |
| B�� | pH=7 ��NH4Cl��NH3•H2O�Ļ����Һ��c��Cl-����c��NH4+����c��H+��=c��OH-�� | |
| C�� | Ũ�Ⱦ�Ϊ0.1 mol•L-1��NH3•H2O��NH4Cl��Һ�������Ϻ�ļ�����Һ�У�c��NH4+����c��Cl-����c��NH3•H2O����c��OH-����c��H+�� | |
| D�� | 0.1 mol•L-1�İ�ˮ��0.05 mol•L-1�� H2SO4��Һ�������Ϻ�������Һ�У�2c��NH4+��+2c��NH3•H2O��=c��SO42-�� |


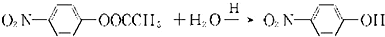 +CH3COOH��
+CH3COOH��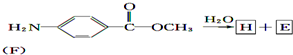
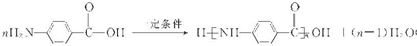 ��
�� �ṹ��
�ṹ�� ��
��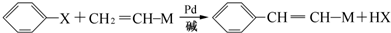
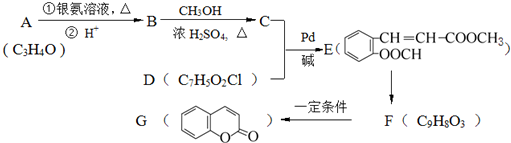

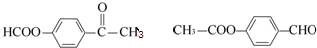
 ���������㶹�ص����Ʒ����������㶹�غ�����һ��ͬ���칹�壨
���������㶹�ص����Ʒ����������㶹�غ�����һ��ͬ���칹�壨 ����Ҫ�õ����Լ��У�NaOH��Һ��ϡ���ᡢ�Ȼ�����Һ
����Ҫ�õ����Լ��У�NaOH��Һ��ϡ���ᡢ�Ȼ�����Һ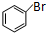 +CH2�TCHCOOCH3$��_{��}^{Pd}$
+CH2�TCHCOOCH3$��_{��}^{Pd}$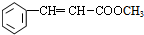 +HBr��
+HBr��