��Ŀ����
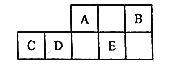
����Ŀ��A��B��C��D��E���ֶ�����Ԫ�������ڱ���λ����ͼ��ʾ��
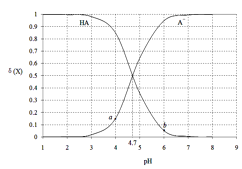
��֪��A��Bԭ�ӵ�������֮�͵���E����������
�ش��������⣺
��1��B�������ӽṹʾ��ͼΪ__��
��2��Dλ�ڵ�__����__�塣
��3����A��B��D��E����̬�⻯���У��ȶ���������__���ѧʽ����
��4����ҵ��ұ��C�ĵ��ʣ���Ҫ�������ۼ�M��Na3AlF6����M��B���⻯������C������������Ӧ��ˮ�����ڸ����ºϳɣ�д����ѧ����ʽ__��
��5��28gD������B��������ȫ��Ӧ����һ�����壬�ų�1615kJ������д���Ȼ�ѧ����ʽ__��
���𰸡�![]() �������� ����A SiH4 3Na2CO3+12HF+2Al(OH)3=2Na3AlF6+9H2O+3CO2�� Si(s)+2H2(g)=SiH4(g)��H=-1615kJ/mol
�������� ����A SiH4 3Na2CO3+12HF+2Al(OH)3=2Na3AlF6+9H2O+3CO2�� Si(s)+2H2(g)=SiH4(g)��H=-1615kJ/mol
��������
A��B��C��D��EΪ������Ԫ�أ�����Ԫ�������ڱ���λ��֪��A��Bλ�ڵڶ����ڣ�C��D��Eλ�ڵ������ڣ���A��ԭ������Ϊx����B��ԭ������Ϊx+2��D��ԭ������Ϊx+7��C��ԭ������Ϊx+6��E��ԭ������Ϊx+9��A��Bԭ�ӵ�������֮�͵���E������������x+x+2=x+9�����x=7������A��NԪ�أ���B��FԪ�أ�C��AlԪ�أ�D��Si��E��S���ٽ��ԭ�ӽṹ�������
���ݷ�����֪��A��NԪ�أ���B��FԪ�أ�C��AlԪ�أ�D��Si��E��S��
(1)����9��Ԫ�أ������ӵ����ӵ����ӽṹʾ��ͼΪ![]() ��
��
(2)D�ǹ裬λ�ڵ������ڣ��ڢ�A��
(3)A��B��D��E����̬�⻯��ֱ�ΪNH3��HF, SiH4��H2S���ǽ�����Խǿ����̬�⻯����ȶ���Խǿ���ǽ�����F>N>S>Si���ȶ���������SiH4��
(4)�������̼���ƣ�����������Ӧ���������������ƣ�ˮ�Ͷ�����̼����ѧ����ʽΪ3Na2CO3+12HF+2Al(OH)3=2Na3AlF6+9H2O+3CO2����
(5)��ͷ�����Ӧ�����ķ��������壬��ѧ����ʽΪSi+2H2=SiH4��28g������ʵ���Ϊn=![]() =
=![]() =1mol���ų�1615kJ���������Ȼ�ѧ����ʽΪSi(s)+2H2(g)=SiH4(g)��H=-1615kJ/mol��
=1mol���ų�1615kJ���������Ȼ�ѧ����ʽΪSi(s)+2H2(g)=SiH4(g)��H=-1615kJ/mol��

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�