ЬтФПФкШн
ЁОЬтФПЁПжаКЭЕЮЖЈЪЧЛЏбЇЖЈСПЪЕбщжЎвЛЁЃФГбЇЩњгћгУвбжЊЮяжЪЕФСПХЈЖШЕФбЮЫсРДВтЖЈЮДжЊЮяжЪЕФСПХЈЖШЕФЧтбѕЛЏФЦШмвКЃЌЧыЬюаДЯТСаПеАзЃК
ЃЈ1ЃЉдкжаКЭЕЮЖЈЕФЙ§ГЬжагаШчЯТВйзїЃКЂйгУБъзМШмвКШѓЯДЕЮЖЈЙм ЂкЭљЕЮЖЈЙмФкзЂШыБъзМШмвК ЂлМьВщЕЮЖЈЙмЪЧЗёТЉЫЎ ЂмЕЮЖЈЃЌдђдкВйзїЙ§ГЬжае§ШЗЕФЫГађЪЧ__________________ЁЃЃЈаДађКХЃЉ
ЃЈ2ЃЉХХШЅМюЪНЕЮЖЈЙмжаЦјХнЕФЗНЗЈгІВЩгУШчЭМЫљЪОВйзїжаЕФ________ЃЌШЛКѓЧсЧсМЗбЙВЃСЇЧђЪЙМтзьВПЗжГфТњМювКЁЃ

ЃЈ3ЃЉбЁгУЕФжИЪОМСЪЧ_____________________ ЁЃ(aЁЂЪЏШя bЁЂЗгЬЊ)
ЃЈ4ЃЉгУБъзМЕФбЮЫсШмвКЕЮЖЈД§ВтЕФЧтбѕЛЏФЦШмвКЪБЃЌзѓЪжАбЮеЫсЪНЕЮЖЈЙмЕФЛюШћЃЌгвЪжвЁЖЏзЖаЮЦПЃЌблОІзЂЪг______________________ЁЃ
ЃЈ5ЃЉЯТСаВйзїжаПЩФмЪЙЫљВтЧтбѕЛЏФЦШмвКЕФХЈЖШЪ§жЕЦЋЕЭЕФЪЧ_______________ЁЃ
AЃЎЫсЪНЕЮЖЈЙмЮДгУБъзМбЮЫсШмвКШѓЯДОЭжБНгзЂШыБъзМбЮЫсШмвК
BЃЎЕЮЖЈЧАЪЂЗХЧтбѕЛЏФЦШмвКЕФзЖаЮЦПгУеєСѓЫЎЯДОЛКѓУЛгаИЩдя
CЃЎЫсЪНЕЮЖЈЙмдкЕЮЖЈЧАгаЦјХнЃЌЕЮЖЈКѓЦјХнЯћЪЇ
DЃЎЖСШЁбЮЫсЬхЛ§ЪБЃЌПЊЪМбіЪгЖСЪ§ЃЌЕЮЖЈНсЪјЪБИЉЪгЖСЪ§
ЃЈ6ЃЉЧыИљОнЯТБэжаЪ§ОнМЦЫуИУЧтбѕЛЏФЦШмвКЕФЮяжЪЕФСПХЈЖШЃКc(NaOH)ЃН ________________ЁЃЃЈОЋШЗЕНаЁЪ§ЕуКѓЫФЮЛЃЉ
ЕЮЖЈДЮЪ§ | Д§ВтЧтбѕЛЏФЦШмвКЕФЬхЛ§/ mL | 0.1000 mol/L бЮЫсЕФЬхЛ§/ mL | ||
ЕЮЖЈЧАПЬЖШ | ЕЮЖЈКѓПЬЖШ | ШмвКЬхЛ§/ mL | ||
ЕквЛДЮ | 25.00 | 0.00 | 26.10 | 26.10 |
ЕкЖўДЮ | 25.00 | 2.00 | 28.08 | 26.08 |
ЕкШ§ДЮ | 25.00 | 0.22 | 26.34 | 26.12 |
ЃЈ7ЃЉЕЮЖЈжеЕуЕФХаЖЈвРОнЪЧ_____________________________________________ЁЃ
ЁОД№АИЁПЂлЂйЂкЂм Бћ b зЖаЮЦПФкШмвКбеЩЋЕФБфЛЏ D 0.1044mol/L ЕЮШызюКѓвЛЕЮбЮЫсЪБЃЌШмвКЧЁКУгЩКьЩЋБфЮЊЮоЩЋЃЌЧвАыЗжжгФкВЛЛжИДЕНдРДЕФбеЩЋ
ЁОНтЮіЁП
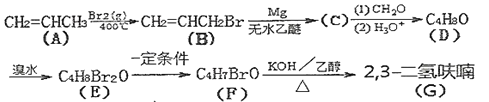
ЃЈ1ЃЉжаКЭЕЮЖЈЕФВйзїВНжшЮЊЃКЂлМьВщЕЮЖЈЙмЪЧЗёТЉЫЎЃЌЂйгУБъзМШмвКШѓЯДЕЮЖЈЙмЃЌЂкЭљЕЮЖЈЙмФкзЂШыБъзМШмвКЃЌЂмЕЮЖЈЃЌЫљвде§ШЗЕФЕЮЖЈЫГађЮЊЃКЂлЂйЂкЂмЃЌЙЪД№АИЮЊЃКЂлЂйЂкЂмЃЛ
ЃЈ2ЃЉХХШЅМюЪНЕЮЖЈЙмжаЦјХнЕФЗНЗЈгІВЩгУБћЃЌШЛКѓЧсЧсМЗбЙВЃСЇЧђЪЙМтзьВПЗжГфТњМювКЃЌЙЪД№АИЮЊЃКБћЃЛ
ЃЈ3ЃЉбЮЫсКЭЧтбѕЛЏФЦЧЁКУЗДгІШмвКГЪжаадЃЌПЩбЁдёЗгЬЊЛђМзЛљГШзїжИЪОМСЃЌЙЪбЁbЃЌЙЪД№АИЮЊЃКbЃЛ
ЃЈ4ЃЉЫсМюжаКЭЕЮЖЈЪБЃЌблОІвЊзЂЪгзЖаЮЦПФкШмвКЕФбеЩЋБфЛЏЃЌЙЪД№АИЮЊЃКзЖаЮЦПжаШмвКбеЩЋЕФБфЛЏЃЛ
ЃЈ5ЃЉAЯюЁЂЫсЪНЕЮЖЈЙмЮДгУБъзМбЮЫсШмвКШѓЯДОЭжБНгзЂШыБъзМбЮЫсШмвКЃЌШмвКБЛЯЁЪЭЃЌБъзМвКЕФХЈЖШЦЋаЁЃЌдьГЩVЃЈБъзМЃЉЦЋДѓЃЌВтЖЈcЃЈД§ВтЃЉЦЋДѓЃЌЙЪAДэЮѓЃЛ
BЯюЁЂЕЮЖЈЧАЪЂЗХЧтбѕЛЏФЦШмвКЕФзЖаЮЦПгУеєСѓЫЎЯДОЛКѓУЛгаИЩдяЃЌД§ВтвКЕФЮяжЪЕФСПВЛБфЃЌЖдVЃЈБъзМЃЉЮогАЯьЃЌВтЖЈcЃЈД§ВтЃЉЮогАЯьЃЌЙЪBДэЮѓЃЛ
CЯюЁЂЫсЪНЕЮЖЈЙмдкЕЮЖЈЧАгаЦјХнЃЌЕЮЖЈКѓЦјХнЯћЪЇЃЌдьГЩVЃЈБъзМЃЉЦЋДѓЃЌВтЖЈcЃЈД§ВтЃЉЦЋДѓЃЌЙЪCДэЮѓЃЛ
DЯюЁЂШЁбЮЫсЬхЛ§ЪБЃЌПЊЪМбіЪгЖСЪ§ЃЌЕЮЖЈНсЪјЪБИЉЪгЖСЪ§ЃЌдьГЩVЃЈБъзМЃЉЦЋаЁЃЌВтЖЈcЃЈД§ВтЃЉЦЋЕЭЃЌЙЪDе§ШЗЁЃ
ЙЪбЁDЃЌЙЪД№АИЮЊЃКDЃЛ
ЃЈ6ЃЉШ§ДЮЕЮЖЈЯћКФбЮЫсЕФЬхЛ§ЗжБ№ЮЊ26.10mLЃЌ26.08mLЃЌ26.12mLЃЌШ§зщЪ§ОнОљгааЇЃЌЦНОљЯћКФVЃЈбЮЫсЃЉ=26.10mLЃЌгЩЛЏбЇЗНГЬЪНПЩЕУЃК0.02610LЁС0.1000mol/L=0.025LЁСcЃЈNaOHЃЉЃЌдђcЃЈNaOHЃЉ= =0.1044mol/L,ЙЪД№АИЮЊЃК0.1044mol/LЃЛ
ЃЈ7ЃЉЕЮЖЈжеЕуЕФХаЖЈвРОнЪЧШмвКбеЩЋгЩЛЦЩЋЭЛБфЮЊГШЩЋЃЌЧвАыЗжжгФкВЛЭЪЩЋЃЌЙЪД№АИЮЊЃКШмвКбеЩЋгЩЛЦЩЋЭЛБфЮЊГШЩЋЃЌЧвАыЗжжгФкВЛЭЪЩЋЁЃ

ЁОЬтФПЁПвбжЊВнЫсФјОЇЬх(NiC2O4ЁЄ2H2O)ФбШмгкЫЎЃЌЙЄвЕЩЯДгЗЯФјДпЛЏМС(жївЊГЩЗжЮЊNiЃЌКЌгавЛЖЈСПЕФAl2O3ЁЂFeOЁЂSiO2ЁЂCaOЕШ)жЦБИВнЫсФјОЇЬхЕФСїГЬШчЭМЫљЪОЃК

вбжЊЃКЂйЯрЙиН№ЪєРызгЩњГЩЧтбѕЛЏЮяГСЕэЕФpHШчЯТБэЃК
Н№ЪєРызг | Fe3+ | Fe2+ | Al3+ | Ni2+ |
ПЊЪМГСЕэЕФpH | 1.1 | 5.8 | 3.0 | 6.8 |
ЭъШЋГСЕэЕФpH | 3.2 | 8.8 | 5.0 | 9.5 |
ЂкKsp(CaF2)=1.46ЁС10Ѓ10
ЂлЕБФГЮяжЪХЈЖШаЁгк1.0ЁС10Ѓ5molЁЄLЃ1ЪБЃЌЪгЮЊЭъШЋГСЕэЁЃ
ЃЈ1ЃЉЧыаДГівЛжжФмЬсИпЁАЫсНўЁБЫйТЪЕФДыЪЉЃК_________________________________ЁЃ
ЃЈ2ЃЉЪдМСaЪЧвЛжжТЬЩЋбѕЛЏМСЃЌаДГіЁАбѕЛЏЁБЪБЗДгІЕФРызгЗНГЬЪН______________ЃЎ
ЃЈ3ЃЉpHЕФЕїПиЗЖЮЇЮЊ__________ЃЌ
ЃЈ4ЃЉаДГіЁАГСИЦЁБЪБЕФРызгЗДгІЗНГЬЪНЃК_________________________________ЁЃЕБCa2+ГСЕэЭъШЋЪБЃЌШмвКжаFЃХЈЖШЕФзюаЁжЕЮЊ___________molЁЄLЃ1(аДГіМЦЫуЪНМДПЩ)ЁЃжЄУїNi2+вбОГСЕэЭъШЋЕФЪЕбщВйзїМАЯжЯѓЪЧ_________ЁЃ
ЃЈ5ЃЉВйзїaЕФФкШнЪЧ___________________________
ЁОЬтФПЁПЯТБэжаЂй~ЂпБэЪОдЊЫижмЦкБэЕФВПЗждЊЫиЁЃ
IA | IIA | IIIA | IVA | VA | VIA | VIIA | |
1 | |||||||
2 | Ђй | Ђк | Ђл | ||||
3 | Ђм | Ђн | Ђо | Ђп |
ЃЈ1ЃЉЂкдЊЫизюЭтВуЕчзгЪ§БШДЮЭтВуЕчзгЪ§Жр______ИіЃЌИУдЊЫиЕФЗћКХЪЧ_______ЃЛЂпдЊЫиЕФЧтЛЏЮяЕФЕчзгЪНЮЊ____________________ЁЃ
ЃЈ2ЃЉгЩЂйЂлЂмШ§жждЊЫизщГЩЕФЮяжЪЪЧ______________ЃЌДЫЮяжЪЕФЫЎШмвКЯд_____адЁЃ
ЃЈ3ЃЉЂодЊЫиЕФдзгАыОЖДѓгкЂлЕФРэгЩЪЧ____________________________________ЁЃ
ЃЈ4ЃЉЂмдЊЫиЕФзюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮяЕФМюадЧПгкЂндЊЫиЃЌгУвЛИіЛЏбЇЗНГЬЪНРДжЄУїЁЃ________________________________________