��Ŀ����
��̼���ƣ�2Na2CO3��3H2O2����һ�ֶ���;������������������Чɱ�𡰼��� H1N1���С���������֪��̼������һ�ֿ�����ˮ�İ�ɫϸС����״��ĩ��50��ɷֽ⣬��3����ˮ��Һ��pHԼΪ10.5����̼���ƾ���Na2C03��H202��˫�����ʡ�
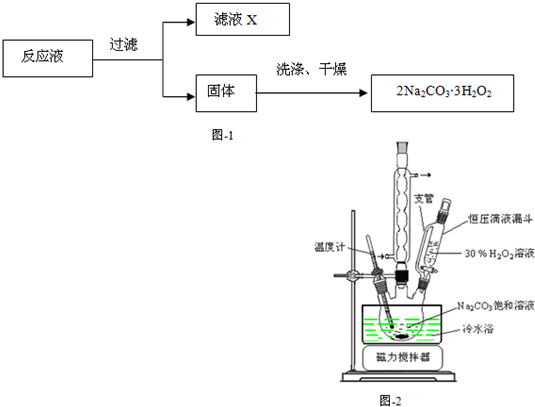
��1��Ϊ̽����̼���Ƶ����ʣ�ijͬѧ���Թ�ȡ������̼������Һ���μӷ�̪��Һ����ʼ���ܹ۲쵽�������� �������������ԭ���� �������ӷ���ʽ��ʾ�����Ȳ����Թܺ��ֿ��ܹ۲쵽 ����
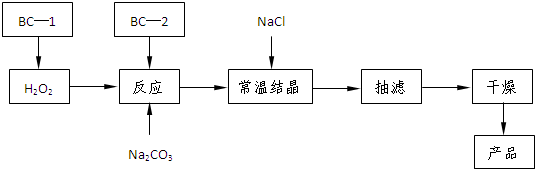
��2����֪��̼������ϡ����ɲ����������塣ij��ѧ����С����������װ�������ϵ��ʵ�顣

��ش��������⣺
�ټ�ͬѧ��װ��I��֤�������������壬B��ʢ��������Ba��OH��2��Һ�����۲쵽�������� ����֤���� �������ɣ�������֤��һ������ķ��� ��
���Ҳ�ѧ������װ����ϣ����ڲⶨ2Na2CO3��3H2O��Ʒ��Na2CO3�ĺ����������������ҵķ���װ��I��II��III������˳���� ����װ����ţ��� B��E��Ӧ�ֱ�ʢ�� �� ��װ��III��ͨ������Ŀ���� ��
��1����Һ��죨1�֣�CO2-3+H2O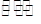 HCO-3+OH-��2�֣���Һ��ɫ��1�֣�
HCO-3+OH-��2�֣���Һ��ɫ��1�֣�
��2���ٲ�����ɫ������1�֣�CO2��1�֣�
ȡ���л��ǵ�ľ������Bƿ�ĵ��ܳ��ڣ���ľ����ȼ˵�����������ɣ�2�֣�
��III��I��II��2�֣�ŨH2SO4��1�֣�NaOH��Һ��1�֣�
��ֹ�����еĶ�����̼��ˮ�������������C�У�1�֣�
��װ��I�еĶ�����̼ȫ������C�У�2�֣�
����������

 ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�| A��ʵ�����Ʊ���̼����ʱ�������ˮԡ���Ʒ�Ӧ�¶� | B����̼����ˮ��Һ�ʼ��ԣ�������Ưϴ��������ɱ���� | C����̼����Ӧ�ܷⱣ�棬�������䰵�� | D����̼���ƿ�ʹ���Ը��������Һ��ɫ�����ų�һ����ɫ���� |



 ��֪������Ӧ 2Na2CO3 ��aq��+3H2O2 ��aq��
��֪������Ӧ 2Na2CO3 ��aq��+3H2O2 ��aq��  2Na2CO3?3H2O2 ��s��
2Na2CO3?3H2O2 ��s��