��Ŀ����
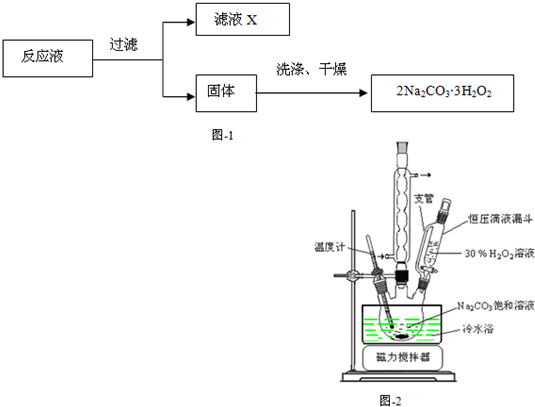
��2013?��������ģ����̼���ƣ�2Na2CO3?3H2O2����һ�ּ�ϴ�ӡ�Ư�ס�ɱ����һ�����ϵƯ�����þ������Na2CO3��H2O2��˫�����ʣ�����ͼ-2װ���Ʊ���̼���ƣ�����ˮԡ�г�ַ�Ӧ��ͼ-1���̿ɻ�ù�̼���Ʋ�Ʒ��
���������գ�
��1����ѹ��Һ©����֧�ܵ�������
��2���Ʊ���̼���ƵĹؼ���
��3������ʹ��̼����ʧЧ��������
a��Na2S b��CH3COOH c��NaHCO3
��̼���Ʋ�Ʒ��������������̼���ƣ������������ⶨ��̼���Ƶ�����������
��4�����ʵ�鲽�裺�ܽ��������Ӧ��
��5��д��������Ӧ�����ӷ���ʽ
��6����Ҫֱ�Ӳⶨ���������У�
��

���������գ�
��1����ѹ��Һ©����֧�ܵ�������
ʹҺ��˳������
ʹҺ��˳������
����2���Ʊ���̼���ƵĹؼ���
���Ʒ�Ӧ�¶�
���Ʒ�Ӧ�¶�
����3������ʹ��̼����ʧЧ��������
C
C
��ѡ���ţ���a��Na2S b��CH3COOH c��NaHCO3
��̼���Ʋ�Ʒ��������������̼���ƣ������������ⶨ��̼���Ƶ�����������
��4�����ʵ�鲽�裺�ܽ��������Ӧ��
����
����
��ϴ��
ϴ��
������
����
������
����
����5��д��������Ӧ�����ӷ���ʽ
Ba2++CO32-=BaCO3��
Ba2++CO32-=BaCO3��
����6����Ҫֱ�Ӳⶨ���������У�
��Ʒ������m1g������������m2g
��Ʒ������m1g������������m2g
������ĸ��ʾ��ע���京�壩����Ʒ�й�̼�������������ı���ʽΪ��314(m1-
| ||
| 102m1 |
314(m1-
| ||
| 102m1 |
��������1����ѹ��Һ©���е�֧����Ҫ�����DZ���ѹǿƽ������Һ�����£�
��2������װ��ͼ������֪�Ʊ���̼���ƵĹؼ��Ƿ�Ӧ���¶ȿ��ƣ�
��3��a��Na2S�ᱻ��̼����������
b�������̼���Ʒ�Ӧ��
c��̼�����ƺ�̼���Ʋ���Ӧ��
��4���ܽ�����Լ�����̼���ƣ����˵õ�����ϴ�Ӻ��������õ�����������
��5��������Ӧ��̼����������ü����Ȼ����γɳ���̼�ᱵ��
��6���������ⶨ��̼��������������Ҫ������Ʒ�����ͳ�������������õ���̼��������������
��2������װ��ͼ������֪�Ʊ���̼���ƵĹؼ��Ƿ�Ӧ���¶ȿ��ƣ�
��3��a��Na2S�ᱻ��̼����������
b�������̼���Ʒ�Ӧ��
c��̼�����ƺ�̼���Ʋ���Ӧ��
��4���ܽ�����Լ�����̼���ƣ����˵õ�����ϴ�Ӻ��������õ�����������
��5��������Ӧ��̼����������ü����Ȼ����γɳ���̼�ᱵ��
��6���������ⶨ��̼��������������Ҫ������Ʒ�����ͳ�������������õ���̼��������������
����⣺��1����ѹ��Һ©���е�֧����Ҫ�����DZ���ѹǿƽ������Һ�����£��ʴ�Ϊ��ʹҺ��˳�����£�
��2������ͼ-2װ���Ʊ���̼���ƣ�����ˮԡ�г�ַ�Ӧ��ͼ-1���̿ɻ�ù�̼���Ʋ�Ʒ�����Թؼ��ǿ��Ʒ�Ӧ���¶ȣ��ʴ�Ϊ�����Ʒ�Ӧ�¶ȣ�
��3��a��Na2S�ᱻ��̼����������ʹ��̼����ʧЧ����a�����ϣ�
b�������̼���Ʒ�Ӧ��ʹ��̼����ʧЧ����b�����ϣ�
c��̼�����ƺ�̼���Ʋ���Ӧ����c���ϣ�
�ʴ�Ϊ��C��
��4����̼���Ʋ�Ʒ��������������̼���ƣ������������ⶨ��̼���Ƶ������������Ȱ���Ʒ�ܽ⣬�����Ȼ�������̼���ƣ����˵õ�������ϴ�ӡ������������м��㣻
�ʴ�Ϊ�����ˡ�ϴ�ӡ����������
��5����Ʒ�е�̼���Ƽ����Ȼ�������Һ�����ӳ���̼������ӣ���Ӧ�����ӷ���ʽΪ��Ba2++CO32-=BaCO3�����ʴ�Ϊ��Ba2++CO32-=BaCO3����
��6��ʵ�������ֱ�Ӳⶨ��������Ϊ��Ʒ������m1g������������m2g���������ʵ���=
�����̼�������ʵ���Ϊx�����е�̼�������ʵ���Ϊy������̼Ԫ���غ�õ�2x+y=
314x+106y=m1
x=
mol��
��̼������������=
=
�ʴ�Ϊ����Ʒ������m1g������������m2g��
��
��2������ͼ-2װ���Ʊ���̼���ƣ�����ˮԡ�г�ַ�Ӧ��ͼ-1���̿ɻ�ù�̼���Ʋ�Ʒ�����Թؼ��ǿ��Ʒ�Ӧ���¶ȣ��ʴ�Ϊ�����Ʒ�Ӧ�¶ȣ�
��3��a��Na2S�ᱻ��̼����������ʹ��̼����ʧЧ����a�����ϣ�
b�������̼���Ʒ�Ӧ��ʹ��̼����ʧЧ����b�����ϣ�
c��̼�����ƺ�̼���Ʋ���Ӧ����c���ϣ�
�ʴ�Ϊ��C��
��4����̼���Ʋ�Ʒ��������������̼���ƣ������������ⶨ��̼���Ƶ������������Ȱ���Ʒ�ܽ⣬�����Ȼ�������̼���ƣ����˵õ�������ϴ�ӡ������������м��㣻
�ʴ�Ϊ�����ˡ�ϴ�ӡ����������
��5����Ʒ�е�̼���Ƽ����Ȼ�������Һ�����ӳ���̼������ӣ���Ӧ�����ӷ���ʽΪ��Ba2++CO32-=BaCO3�����ʴ�Ϊ��Ba2++CO32-=BaCO3����
��6��ʵ�������ֱ�Ӳⶨ��������Ϊ��Ʒ������m1g������������m2g���������ʵ���=
| m2 |
| 197g/mol |
| m2 |
| 197 |
314x+106y=m1
x=
m1-
| ||
| 102 |
��̼������������=
| x��314 |
| a |
314(m1-
| ||
| 102m1 |
�ʴ�Ϊ����Ʒ������m1g������������m2g��
314(m1-
| ||
| 102m1 |
���������⿼�����Ʊ�ʵ����̵ķ����жϣ�װ��ͼ�ķ���Ӧ�ã�ע���̼���Ƶ���ɺͻ�����гɷֵ������������㷽������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
