��Ŀ����
����Ŀ���±���Ԫ�����ڱ���һ���֣����е����ֱ�ʾһ�ֶ�����Ԫ�����ش��������⣺

��1����������ԭ�ӽṹʾ��ͼ��____________________��
��2���ۡ��ܡ��ߡ�������Ԫ�����γɵ���̬�⻯�������ȶ�����__________���ѧʽ����
��3������Ԫ�����ڱ��е�λ����____________________________��
��4���ࡢ��Ԫ�ص�����������Ӧˮ��������ԣ�____>____���û�ѧʽ��ʾ����_________
��5�����ۡ���������ɵĻ�����������ѧ������Ϊ__________������ʽΪ____________��
��6���ܡ��ݡ�����ԭ�Ӱ뾶�ɴ�С��˳���ǣ�____>____>____����Ԫ�ط��ű�ʾ����__________
��7������������������Һ��Ӧ�Ļ�ѧ����ʽ��__________________________________��
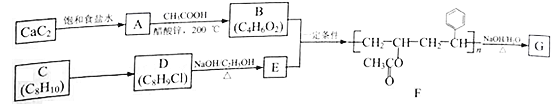
��8����֪������A��B��C��D��E��F��G�͵��ʼס�������Ԫ�ؾ�Ϊ�ϱ���Ԫ����ɡ�
��A��Ũ��Һ����ܷ�������ͼ��ʾ�ķ�Ӧ��
�����dz����ĺ�ɫ���嵥�ʣ���Ϊ���������ṩ������
�����dz�������ɫ���嵥�ʡ�
��B����ɫ�д̼�����ζ�����壬����Ҫ�Ĵ�����Ⱦ��֮һ��
�������£�C��һ����ɫҺ�塣

�ش����⣺
��д���������ʵĻ�ѧʽ��A_____��E_____��G_____��
��д�����з�Ӧ�Ļ�ѧ����ʽ��
C��E��F����________________________________��
B��C������A________________________________��
���𰸡�![]() HF �ڶ�����IVA�� HClO4 H2SO4 ���Ӽ������ۼ�
HF �ڶ�����IVA�� HClO4 H2SO4 ���Ӽ������ۼ� ![]() Na Al F 2Al��2OH����2H2O��2AlO2����3H2�� H2SO4 Na2O2 Na2CO3 2Na2O2��2H2O��4NaOH��O2�� 2SO2��O2��2H2O��2H2SO4
Na Al F 2Al��2OH����2H2O��2AlO2����3H2�� H2SO4 Na2O2 Na2CO3 2Na2O2��2H2O��4NaOH��O2�� 2SO2��O2��2H2O��2H2SO4
��������
����Ԫ�����ڱ�֪���٢ڢۢܢݢޢߢ�����������Ԫ�طֱ�ΪC��N��O��F��Na��Al��P��S��Cl��H��
��1������OԪ�أ�Oԭ��������=���������=8��K��2�����ӡ�L��6�����ӣ�ԭ�ӽṹʾ��ͼ��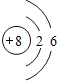 ���ʴ�Ϊ��
���ʴ�Ϊ��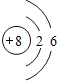 ��
��
��2���ۡ��ܡ��ߡ�������Ԫ�طֱ���O��F��P��S���ǽ�����Խǿ����̬�⻯��Խ�ȶ������зǽ�������ǿ��Ԫ����F�����HF���ȶ����ʴ�Ϊ��HF��
��3������CԪ�أ���Ԫ�����ڱ��е�λ���ǵڶ�����IVA�壬�ʴ�Ϊ���ڶ�����IVA�壻
��4���ࡢ��Ԫ�طֱ���S��Cl���ǽ�����Cl��S���ǽ�����Խǿ������������Ӧˮ���������Խǿ��������ԣ�HClO4��H2SO4���ʴ�Ϊ��HClO4��H2SO4��
��5���ۡ��ݺ͢�Ԫ�طֱ���O��Na��H���ɢۡ��ݺ͢���ɵĻ�������NaOH��NaOH�����ӻ����������ѧ������Ϊ���Ӽ����ۼ�������ʽΪ��![]() ���ʴ�Ϊ�����Ӽ������ۼ���
���ʴ�Ϊ�����Ӽ������ۼ���![]() ��
��
��6���ܡ��ݡ��ֱ���F��Na��Al������ͬ����������ԭ�Ӱ뾶��С��ԭ�Ӱ뾶��Na��Al��Cl��ͬ�������϶���ԭ�Ӱ뾶������ԭ�Ӱ뾶��Cl��F�����ԭ�Ӱ뾶��Na��Al��F���ʴ�Ϊ��Na��Al��F��
��7������AlԪ�أ�Al������������Һ��Ӧ�����ӷ���ʽΪ2Al��2OH����2H2O��2AlO2����3H2����
��8�����ɷ�Ӧ��ϵͼ��֪�����dz����ĺ�ɫ���嵥�ʣ���Ϊ���������ṩ���ܣ���֪����C��B����ɫ�д̼�����ζ�����壬����Ҫ�Ĵ�����Ⱦ��֮һ��ΪSO2���壬���AΪŨ������Һ�������£�C��һ����ɫҺ����ˮ��DΪCO2�����������������ˮ��Ӧ�������ᣬ����ΪO2��ˮ��������̼����E��Ӧ����������˵��EΪNa2O2����FΪNaOH��GΪNa2CO3���ʴ�Ϊ��H2SO4��Na2O2��Na2CO3��
��ˮ�������Ʒ�Ӧ�����������ƺ���������ѧ����ʽΪ��2Na2O2+2H2O=4NaOH+O2�������������������ˮ��Ӧ�������ᣬ��ѧ����ʽΪ��2SO2+O2+2H2O=2H2SO4���ʴ�Ϊ��2Na2O2+2H2O=
4NaOH+O2����2SO2+O2+2H2O=2H2SO4��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��������(CH3OCH3)����Ϊ21���͵�����ȼ�ϣ���CO��H2Ϊԭ��������������Ҫ��������������Ӧ��
��1���ù��յ��ܷ�ӦΪ3CO(g)��3H2(g)![]() CH3OCH3(g)��CO2(g) ��H��_________����ѧƽ�ⳣ��K��______________(�ú�K1��K2��K3�Ĵ���ʽ��ʾ)��
CH3OCH3(g)��CO2(g) ��H��_________����ѧƽ�ⳣ��K��______________(�ú�K1��K2��K3�Ĵ���ʽ��ʾ)��
��ѧ��Ӧ����ʽ | ��ѧƽ�ⳣ�� | |
��CO(g)��2H2(g) | ��H1=-99 kJmol-1 | K1 |
��2CH3OH(g) | ��H2����24 kJmol-1 | K2 |
��CO(g)��H2O(g) | ��H3����41 kJmol-1 | K3 |
��2��ij�¶��£���8.0molH2��4.0molCO�����ݻ�Ϊ2L���ܱ������У�������Ӧ��4H2(g)+2CO(g) ![]() CH3OCH3(g)+H2O(g)��10 ���Ӻ�Ӧ��ƽ�⣬��ö����ѵ��������Ϊ25%������H2��ʾ�ķ�Ӧ����Ϊ_________��CO��ת����Ϊ________��
CH3OCH3(g)+H2O(g)��10 ���Ӻ�Ӧ��ƽ�⣬��ö����ѵ��������Ϊ25%������H2��ʾ�ķ�Ӧ����Ϊ_________��CO��ת����Ϊ________��
��3�����д�ʩ�У������CH3OCH3���ʵ���________��
A������������� B�������¶� C�����ø�Ч����D������ѹǿ
��4���ù����з�Ӧ�۵ķ��������CH3OCH3�IJ��ʣ�ԭ����________________��
����Ŀ�����������ɢϵ�������ͺ�ˮ�γɵĻ��Һ�ں�����ɳ��ʳ��ˮ�����е�(I2)���Ȼ�����Һ���Ҷ����ͱ����������Һ(�Ҷ����ͱ������IJ����������ʼ���)��
���� | �۵�/�� | �е�/�� | �ܶ�/gcm-3 | �ܽ��� |
�Ҷ��� | 11.5 | 198 | 1.11 | ������ˮ���Ҵ� |
������ | 17.9 | 290 | 1.26 | �ܸ�ˮ���ƾ�������Ȼ��� |
������ͼ��ʾ�������������ϸ����Һ�������ͷ������ܶ�Ӧ���ǣ� ��

A. ����c����ҺB. ����a����ȡ
C. ����c����ȡD. ����a������
����Ŀ��һ���¶�ʱ�����ݻ�Ϊ 2 L ���ܱ������г���һ������ SO2(g)�� O2(g)��������Ӧ��2SO2(g) + O2(g) ![]() 2SO3(g) ��H = -196 kJ/mol��һ��ʱ���Ӧ�ﵽƽ��״̬����Ӧ�����вⶨ�IJ������������ʾ��
2SO3(g) ��H = -196 kJ/mol��һ��ʱ���Ӧ�ﵽƽ��״̬����Ӧ�����вⶨ�IJ������������ʾ��
��Ӧʱ��/min | n(SO2)/mol | n(O2)/mol |
0 | 2 | 1 |
5 | 1.2 | |
10 | 0.4 | |
15 | 0.8 |
����˵������ȷ����
A. ǰ5 min��ƽ����Ӧ����Ϊ��(SO2) = 0.08 mol/(L��min)
B. �����¶Ȳ��䣬��ƽ�����������ٳ��� 0.2 mol SO2(g)��0.2 mol SO3(g)ʱ������> ����
C. ��ͬ�¶��£���ʼʱ�������г��� 1.5 mol SO3(g)���ﵽƽ��״̬ʱ SO3 ��ת����Ϊ40%
D. ���������������䣬����ʼʱ�������г��� 2 mol SO3(g)���ﵽƽ��״̬ʱ���� 78.4 kJ ������