��Ŀ����
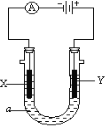
����Ŀ����֪��TKʱ��Ca(OH)2��CaWO4������ˮ��pCa����lgc(Ca2��)��pX����lgc(WO42-)��lgc(OH��)��TKʱCaWO4��Ca(OH)2�ij����ܽ�ƽ��������ͼ��ʾ��

��1������Na2WO4��Һ�м��뱥��ʯ��ˮ������Ϊ__�����ӷ���ʽΪ��__��
��Na2WO4��Һ��ʯ�����ϣ������������CaWO4����÷�Ӧ��ƽ�ⳣ��K��__��
��2����֪Ksp(BaSO4)��1.1��10��10��Ksp(BaMoO4)��4.0��10��8�������ƾ���(Na2MoO4��2H2O)�����͵Ľ�����ʴ������������������Һ�������������������������ʣ��ɼ���Ba(OH)2�����ȥSO42-(��Һ����仯����)����BaMoO4��ʼ����ʱ����Һ�е�![]() ��__(�������2λ��Ч����)��
��__(�������2λ��Ч����)��
���𰸡����ɰ�ɫ���� Ca2����WO42-=CaWO4�� 100 3.6��102
��������
�ɱ������ݿ�֪��Ksp(CaWO4)��1��10��10��Ksp[Ca(OH)2]��1��10��8����Ȼ��Ca(OH)2���ܽ�ȱ�CaWO4�����ܶȻ��ı���ʽ������ؼ�����жϡ�
��1��������CaWO4���ܽ�ȱ�Ca(OH)2С����һ��Ũ�ȵ�Na2WO4��Һ�м��뱥��ʯ��ˮ�����������ܽ�Ƚ�С��CaWO4����������Ϊ���ɰ�ɫ���������ӷ���ʽΪ��Ca2����WO42-=CaWO4����
��Na2WO4��Һ��ʯ�����ϣ������������CaWO4���ù��̷���������ת�������ӷ���ʽΪCa(OH)2��WO42- CaWO4+2OH-����÷�Ӧ��ƽ�ⳣ��K��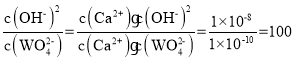 ��
��
��2����֪Ksp(BaSO4)��1.1��10��10��Ksp(BaMoO4)��4.0��10��8����������������Һ�������������������������ʣ��ɼ���Ba(OH)2�����ȥSO42-(��Һ����仯����)����BaMoO4��ʼ����ʱ��BaSO4�ѳ�����ȫ��ͬ�����ƣ���Һ�е�![]() ��[Ksp(BaMoO4)]��[Ksp(BaSO4)]=��4.0��10��8������1.1��10��10��=3.6��102��
��[Ksp(BaMoO4)]��[Ksp(BaSO4)]=��4.0��10��8������1.1��10��10��=3.6��102��

����Ŀ�����и�������������ܴﵽԤ��ʵ��Ŀ�ĵ���
ѡ�� | ���������� | ʵ��Ŀ�� |
A | �����������Թ��У��������û | ��֤����������ʴ |
B | ��NaBr��Һ�е���������ˮ�ͱ��������ã���Һ�ϲ�ʳȺ�ɫ | ֤��Br����ԭ��ǿ��Cl�� |
C | ���Ȼ���������������������ˮ�� | ����FeCl2��Һ |
D | �������Ȼ������ʵ�����ͨ��װ�б���NaHCO3��Һ��ϴ��ƿ | ��ȥ�����е�HCl���� |
A. A B. B C. C D. D
����Ŀ���¶�ΪT1ʱ���������ݻ���Ϊ1L�ĺ����ܱ������н�������Ӧ��2NO2(g)![]() 2NO(g)+O2(g)����������±���ʾ������˵����������� ��
2NO(g)+O2(g)����������±���ʾ������˵����������� ��
���� ��� | ���ʵ���ʼŨ�� (mol��L-1) | ���ʵ�ƽ��Ũ�� (mol��L-1) | ||
c(NO2) | c(NO) | c(O2) | c(O2) | |
�� | 0.6 | 0 | 0 | 0.2 |
�� | 0.3 | 0.5 | 0.2 | |
�� | 0 | 0.5 | 0.35 | |
A. �������з�����Ӧ��ƽ�ⳣ��Ϊ0.8 B. �������з�����Ӧ����ʼ����v��>v��
C. �ﵽƽ��ʱ����������![]() >1 D. �ﵽƽ��ʱ�����������������е���ѹǿ֮��Ϊ16��17
>1 D. �ﵽƽ��ʱ�����������������е���ѹǿ֮��Ϊ16��17
����Ŀ��ij���γ�������Cr(��)��ˮ����ȡCr2O3��һ�ֹ���������ͼ��ʾ��
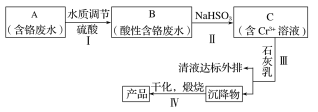
��֪��Ksp[Cr(OH)3]��6.3��10��31
Ksp[Fe(OH)3]��2.6��10��39
Ksp[Fe(OH)2]��4.9��10��17
��1��������У������ķ�ӦΪ2H����2CrO42-![]() Cr2O72-��H2O��B�к���Ԫ�ص�������___(�����ӷ���)��
Cr2O72-��H2O��B�к���Ԫ�ص�������___(�����ӷ���)��
��2������Һ��Cr3����Ũ�ȡ�1.5mg��L��1ʱ������Ϊ�Ѵ�����ŷű����������Һ��pH��5������Һ�в����ϸ����ŷű�����Ϊ��ʱCr3����Ũ�ȣ�__mg��L��1��
��3����������������ʴ���NaHSO3����ԭ����
������FeSO4��7H2O����ԭ����������вμӷ�Ӧ��������һ����__(�����ӷ���)��
��������м����ԭ����������Ͷ������ͬʱ�������㣬C��Һ��pH��c(Cr2O72-)�Ķ�Ӧ��ϵ���±���ʾ��
pH | 3 | 4 | 5 | 6 |
c(Cr2O72-)/mol��L��1 | 7.02��10��21 | 7.02��10��7 | 1.25��10��3 | 2.21��10��34 |
������ΪpH��6ʱ��c(Cr2O72-)��С��ԭ����Cr2O72-�����϶���ת��ΪCr3��������˵���Ƿ���ȷ��Ϊʲô��___��
������м����ԭ��ʱ��Ϊʹ���ò�Ʒ�к���Ԫ�����ʵĺ��������ܵͣ���Ҫ���Ƶ�������___��
����Ŀ�������ܿ�����ָʾ���ʹ������Ʊ�����ˮ�ܿ�(��Ҫ�ɷ�ΪCo2O3��������Fe2O3��Al2O3��MnO��MgO��CaO��SiO2��)��ȡCoC2O4��2H2O������������ͼ��ʾ��
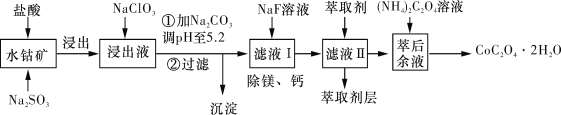
��֪���ٽ���Һ���е���������Ҫ��H����Co2+��Fe2+��Mn2+��Ca2+��Mg2+��Al3+�ȣ�
�����������£�ClO3-��������Co2+��ClO3-ת��ΪCl-��
�۲���������������������ʽ����ʱ��Һ��pH���±���
������ | Fe(OH)3 | Al(OH)3 | Co(OH)2 | Fe(OH)2 | Mn(OH)2 |
��ȫ������pH | 3.7 | 5.2 | 9.2 | 9.6 | 9.8 |
(1)���������м���Na2SO3����ҪĿ����_______________________��
(2)�����Һ�м���NaClO3�����ӷ�Ӧ����ʽ��_____________________��
(3)��֪��������NH3��H2O![]() NH4++OH-��Kb=1.8��10-5
NH4++OH-��Kb=1.8��10-5
H2C2O4![]() H��+HC2O4-��Ka1=5.4��10-2
H��+HC2O4-��Ka1=5.4��10-2
HC2O4-![]() H��+C2O42-��Ka2=5.4��10-5
H��+C2O42-��Ka2=5.4��10-5
�������������(NH4)2C2O4��Һ��pH____7 (����>������<������=��)��
(4)����(NH4)2C2O4��Һ���������壬�ٹ��ˡ�ϴ�ӣ�ϴ��ʱ��ѡ�Լ�_____(����ĸ����)��
A.����ˮ B.����ˮ C.���͵�(NH4)2C2O4��Һ D.ϡ����
(5)��ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ��ʾ����ȡ���������dz�ȥ�����ӣ���ʹ�õ�����pH��Χ��_________(����ĸ����)��
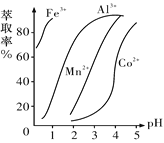
A.2.0��2.5�������� B.3.0��3.5 ������C.4.0��4.5
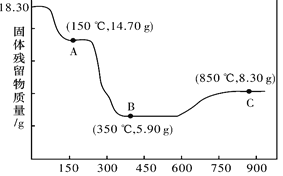
(6)CoC2O4��2H2O�ȷֽ������仯������ͼ��ʾ������600����ǰ�Ǹ����������ȣ�600���Ժ����ڿ����м��ȡ�A��B��C��Ϊ�����C����ʾ����Ļ�ѧʽ��_____________��
����Ŀ����֪N2(g)��3H2(g)![]() 2NH3(g) ��H=92.4 kJ��mol��1�����¶���ͬ���ݻ���Ϊ2 L��3�������ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��£���÷�Ӧ�ﵽƽ��ʱ���й��������£�����˵����ȷ����
2NH3(g) ��H=92.4 kJ��mol��1�����¶���ͬ���ݻ���Ϊ2 L��3�������ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��£���÷�Ӧ�ﵽƽ��ʱ���й��������£�����˵����ȷ����
���� | �� | �� | �� |
��Ӧ��Ͷ���� | 1 mol N2��3 mol H2 | 2 mol N2��6 mol H2 | 2 mol NH3 |
NH3��Ũ�ȣ�mol��L��1�� | c1 | c2 | c3 |
��Ӧ�������仯 | �ų�Q1kJ | �ų�Q2kJ | ����Q3kJ |
��ϵѹǿ��Pa�� | p1 | p2 | p3 |
��Ӧ��ת���� | ��1 | ��2 | ��3 |
A.Q3+92.4c1��92.4B.��2����3<1
C.2p1��2p3<p2D.�ﵽƽ��ʱ��������NH3������������