��Ŀ����
����Ŀ����ͼ������ A~J��һ�������µ�ת����ϵ�����ֲ��P��Ӧ����δ�г���������CΪ����ɫ���壻H��I�����ֳ����Ľ������ʣ�����I�� D��Һ��Ӧ����A��

����д���пհף�
(1)HԪ�������ڱ��е�λ����________��д���ٵ����ӷ�Ӧ����ʽ��_________������E��Һ�������ӵ�����Լ�Ϊ________��
(2)���෴Ӧ������ұ�����۵�Ľ�������MnO2ұ�������̵ķ�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ__________��
(3)SCR��������������β��ʱ���ڴ����������ð�����C��Ӧ����������Ⱦ�����ʡ�������Ӧ�Ļ�ѧ����ʽΪ__________��
(4)F��NaClO��NaOH��Һ��Ӧ�����Ƶ�һ������ɫ����Ч��ˮ��K2FeO4��ÿ����1molFeO42-ʱת��____________mol���ӡ�
���𰸡��������ڢ�A�� 3Fe2++NO3-+4H+=3Fe3++NO��+2H2O KSCN��Һ 3��4 8NH3+6NO2![]() 7N2+12H2O 3
7N2+12H2O 3
��������
H��I�����ֳ����Ľ������ʣ�H��G�ڸ��������·�Ӧ����I��J����H��Al��I��Fe��J��Al2O3��G��Fe�������CΪ����ɫ���壬C��B��O2��Ӧ���ɣ���B��NO��C��NO2��C��H2O��Ӧ������D��HNO3������I��D��Һ��Ӧ����A����A��Fe(NO3)2��E��Fe(NO3)3��E��NaOH��Һ��Ӧ����F��Fe(OH)3��F���ȷֽ����G��GΪFe2O3���ݴ˴��⡣
��1��H��Al��AlԪ�������ڱ��е�λ���ǵ������ڢ�A�壬��Ӧ����Fe2+�����������±�NO3-������ΪFe3+�Ĺ��̣����ӷ�Ӧ����ʽ��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O��E�н�����������Fe3+������Fe3+������Լ�ΪKSCN��Һ����������Һ��ΪѪ��ɫ����֤������Fe3+���ʴ�Ϊ���������ڢ�A�壻3Fe2++NO3-+4H+=3Fe3++NO��+2H2O��KSCN��Һ��
��2�����ȷ�Ӧ������ұ�����۵�Ľ���������MnO2ұ�������̣���Ӧ����ʽΪ4Al+3MnO2![]() 3Mn+2Al2O3���ڷ�Ӧ����������MnO2����ԭ����Al���������뻹ԭ�������ʵ���֮��Ϊ3��4���ʴ�Ϊ��3��4��
3Mn+2Al2O3���ڷ�Ӧ����������MnO2����ԭ����Al���������뻹ԭ�������ʵ���֮��Ϊ3��4���ʴ�Ϊ��3��4��
��3��SCR��������������β��ʱ���ڴ����������ð�����NO2��Ӧ����������Ⱦ������N2��ˮ�����ݵ����غ��ԭ���غ㣬��֪������Ӧ�Ļ�ѧ����ʽΪ8NH3+6NO2![]() 7N2+12H2O���ʴ�Ϊ��8NH3+6NO2
7N2+12H2O���ʴ�Ϊ��8NH3+6NO2![]() 7N2+12H2O
7N2+12H2O
��4��F��Fe(OH)3��Fe(OH)3��NaClO��NaOH��Һ��Ӧ�����Ƶ�һ�֡���ɫ����Ч��ˮ��K2FeO4��Fe(OH)3��FeԪ�صĻ��ϼ�Ϊ+3�ۣ���K2FeO4��FeԪ�صĻ��ϼ�Ϊ+6�ۣ�����ÿ����1molFeO42-ʱת��3mol���ӣ��ʴ�Ϊ��3��

����Ŀ��������һ�ֳ����Ļ������ҵ����Ҳ��һ����ȡ������ͭ�������ԭ�ϡ����з�ͭ����Ҫ����ΪFe�����Ʊ�����������������������̣�
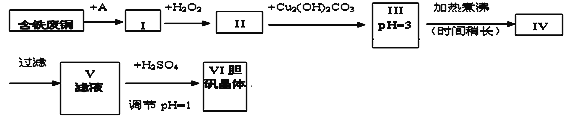
pHֵ���ƿɲο���������
�� �� | ��ʼ����ʱ��pHֵ | ��ȫ����ʱ��pHֵ |
�������� | 2.7 | 3.7 |
���������� | 7.6 | 9.6 |
������ͭ | 5.2 | 6.4 |
������������̻ش��������⣺
��1��A���ʿ�ѡ��_____������ĸ����
a��ϡH2SO4 b��ŨH2SO4/�� c��ŨFeCl3��Һ d��ŨHNO3
��2��I�м�H2O2�����ӷ���ʽ________________________________________��
��3��II�м�Cu2��OH��2CO3��Ŀ����________________________�����ŵ���__________��
��4��III�������ʱ�����Ļ�ѧ��Ӧ�����ӷ���ʽΪ________________________��
��5��V�м�H2SO4����pH=1��Ϊ��____________________________________________��
��6��V��VI�IJ�����_________________________________
��7��ijͬѧ��Ϊ�������������ӵ�A���ʲ������룬�����Ľ�����������__________����θĽ�___________________��