��Ŀ����
����Ŀ����84����Һ������Чɱ�����HIN1�Ȳ�����ijͬѧ������һƿ����¶ʿ���ơ�84����Һ����������������Ϻ�����Һ��װ˵���õ�������Ϣ����84����Һ������25%NaClO��1000 mL���ܶ�1.192g/cm3��ϡ��100��������ȣ���ʹ�á������������Ϣ�����֪ʶ�ش��������⣺
��1��100gij84����Һ��3.55g���������������൱���ò�Ʒ����Ч�Ⱦ���3.55%������100gij84����Һ�к���___gNaClO��
��2��һƿ����¶ʿ���ơ�84����Һ����������տ�����CO2___L����״���������ʡ�
��3����ͬѧ���ġ���¶ʿ���ơ�84����Һ�����䷽������NaClO��������480 mL��25%NaClO������Һ������˵����ȷ����___�����ţ���
A.��ͼ��ʾ�������У��������Dz���Ҫ�ģ��������ֲ�������
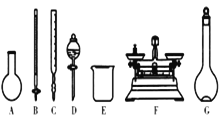
B.����ƿ������ˮϴ����Ӧ��ɲ���������Һ����
C.���ù������ƷNaClO�����ƿ��ܵ��½��ƫ��
D.��Ҫ������NaClO��������Ϊ143 g
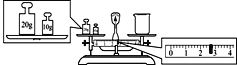
��4��ijͬѧ��������ƽ�����ձ�����������ƽƽ����״̬��ͼ����ͼ�п��Կ������ձ���ʵ������Ϊ___g��
��5������һ�����ʵ���Ũ�ȵ�������Һ�����в������������Ƶ�ϡ�������ʵ���Ũ��ƫ�͵���___(����ĸ)��
A��δ�ָ������¾ͽ���Һע������ƿ�����ж���
B������Ͳ��ȡŨ����ʱ���Ӱ�Һ��
C������ƿ������ˮϴ��δ����
D��δϴ���ձ��Ͳ�����
E������ʱ����Һ��
���𰸡�3.725g 89.6 AC 27.4 BDE
��������
(1). ����Ч�Ⱥ��������������������������������������䶨����:ÿ�˺��������������������൱�ڶ��ٿ�Cl2������������100gij84����Һ��3.55g���������������൱����������Ƶ����ʵ�����������ͬ����m=![]() �õ����ʵ�����
�õ����ʵ�����
��2��c=![]() ����Ũ��������ʵ���Ũ�ȣ����û�ѧ����ʽ�����������̼�����ʵ�����V=n��22.4�����������̼�������
����Ũ��������ʵ���Ũ�ȣ����û�ѧ����ʽ�����������̼�����ʵ�����V=n��22.4�����������̼�������
��3������������Һ�еIJ���������⣻��������һ�����ʵ���Ũ�ȵ���Һ�����Ʋ���ѡ��ʹ�õ�����������c=![]() ��ͨ���жϲ������������ʵ����ʵ���n����Һ���V��Ӱ����������
��ͨ���жϲ������������ʵ����ʵ���n����Һ���V��Ӱ����������
��4����ƽ��������ʱ��ѭ���������ԭ����ƽƽ��ԭ����������������=������������+����������
��5������һ�����ʵ���Ũ�ȵ���Һ�����Ʋ���ѡ��ʹ�õ�����������c=![]() ��ͨ���жϲ������������ʵ����ʵ���n����Һ���V��Ӱ����������
��ͨ���жϲ������������ʵ����ʵ���n����Һ���V��Ӱ����������
(1) ���ݷ����ɵã�100g����Һ�к��ȵ����ʵ���=![]() =
=![]() =0.05mol��������ԭ���غ㣬NaClO�����ʵ���Ϊ0.05mol����NaClO������Ϊ0.05mol��74.5= 3.725g��
=0.05mol��������ԭ���غ㣬NaClO�����ʵ���Ϊ0.05mol����NaClO������Ϊ0.05mol��74.5= 3.725g��
�ʴ�Ϊ3.725g��
(2) ��84����Һ����NaClO �����ʵ���Ũ��=![]() =
=![]() = 4.0mol/L�� n(NaClO)=1L��4.0 mol/L=4.0 mol�����ݷ�ӦCO2+NaClO+H2O�TNaHCO3+HClO������ҪCO2�����ʵ���Ϊn(NaClO)=4.0 mol������״����V(CO2)=4.0 mol��22.4 L/mol=89.6 L��
= 4.0mol/L�� n(NaClO)=1L��4.0 mol/L=4.0 mol�����ݷ�ӦCO2+NaClO+H2O�TNaHCO3+HClO������ҪCO2�����ʵ���Ϊn(NaClO)=4.0 mol������״����V(CO2)=4.0 mol��22.4 L/mol=89.6 L��
�ʴ�Ϊ��89.6��
��3��A������������ƽ����NaClO���壬�����ձ����ܽ�NaClO�����ò��������н������������������ƿ�ͽ�ͷ�ι������ݣ�ͼʾ��A. B. C. D����Ҫ�������貣�����ͽ�ͷ�ιܣ���A��ȷ��
B. ���ƹ�������Ҫ����ˮ�����Ծ�ϴ�Ӹɾ�������ƿ���غ�ɺ���ʹ�ã���B����
C. ����NaClO�����տ����е�H2O��CO2�����ʣ�������ƷNaClO���ܲ��ֱ��ʵ���NaClO���٣����Ƶ���Һ�����ʵ����ʵ�����С�����ƫ�ͣ���C��ȷ��
D. Ӧѡȡ500 mL������ƿ�������ƣ�Ȼ��ȡ��480 mL���ɣ�������ҪNaClO��������0.5 L��4.0 molL1��74.5 gmol1=149 g����D����
�ʴ�Ϊ��AC��
��4����ƽ���̵�����������������������������������������������30g�������������2.6g,���ձ�����������30.0g-2.6g=27.4g��
�ʴ�Ϊ��27.4g��
��5��������Һʱ��c=![]() ��������
��������
A��δ�ָ������¾ͽ���Һע������ƿ�����ж��ݣ���ʹVС��Ũ��ƫ��A����
B������Ͳ��ȡŨ����ʱ���Ӱ�Һ�棬��ʹnƫС��Ũ��ƫС����B��ȷ��
C������ƿ������ˮϴ��δ������滹Ҫ���ݣ�����Ӱ�죬��C����
D��δϴ���ձ��Ͳ���������ʹnƫС��Ũ��ƫС����D��ȷ��
E������ʱ����Һ�棬��ʹVƫ��Ũ��ƫС����E��ȷ��
�ʴ�ΪBDE��

 �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�