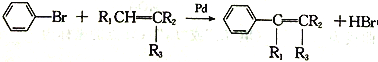��Ŀ����
����Ŀ��I�����Ǽ���ʵ���г��õ�������
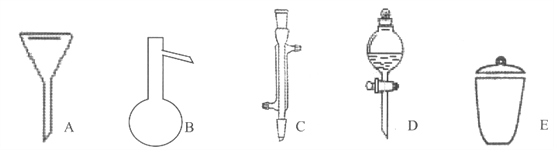
д����������������������ƣ�
A__________��B__________��C__________��D__________��E__________
IIʵ����Ҫ����100 mL 2 mol��L NaCl��Һ����ش��������⣺
��1�����ƹ�������Ҫʹ�õ���Ҫ�������������ձ�������������ͷ�ιܡ���ƽ����Ͳ��__________________��
��2����������ƽ��ȡ�Ȼ��ƹ��壬������Ϊ__________g��
��3��������Ҫ�����������ȷ˳����____________________������ţ���
�ٳ�ȡһ���������Ȼ��ƣ������ձ��У�����������ˮ�ܽ⣻
�ڼ�ˮ��Һ��������ƿ���̶�����1��2����ʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������У�
�۽���Һת�Ƶ�����ƿ�У�
�ܸǺ�ƿ�����������µߵ���ҡ�ȣ�
������������ˮϴ���ձ��ڱںͲ�����2��3�Σ�ϴ��Һת�Ƶ�����ƿ�С�
��4�����ʵ�������ȱ�ٲ���ݣ������������Һ�����ʵ���Ũ��__________ ���ƫ�ߡ���ƫ�͡�����Ӱ�족����
���𰸡� ©�� ������ƿ ������ ��Һ©�� ���� 100 mL����ƿ 11.7 �٢ۢݢڢ� ƫ��
��������I��������ͼ�ο�֪AΪ©����B������ƿ��CΪ�����ܣ�DΪ��Һ©����EΪ�������ʴ�Ϊ��©����������ƿ�������ܣ���Һ©����������
II(1)�ù�������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ���������������ƽ��ҩ�ס��ձ���������������ƿ����ͷ�ιܣ�����100mL2mol/LNaCl��Һ��Ӧѡ��100mL����ƿ�����Ի�ȱ�ٵ�������100mL����ƿ���ʴ�Ϊ��100mL����ƿ��
(2)����100mL2mol/LNaCl��Һ����Ҫ���ʵ�����Ϊ��0.1L��2mol/L��58.5g/mol=11.7g���ʴ�Ϊ��11.7��
(3)�ù�������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ������ǩ��������ȷ�IJ�������Ϊ���٢ۢݢڢܣ��ʴ�Ϊ���٢ۢݢڢܣ�
(4)���ʵ�������ȱ�ٲ���ݣ�����ɲ���������ģ����ʵ����ʵ���ƫС��������Һ�����ʵ���Ũ�� ƫ�ͣ��ʴ�Ϊ��ƫ�͡�