��Ŀ����
4���ϳɰ����ع�ҵ�����������漰��������ת��������ͼ��ʾ��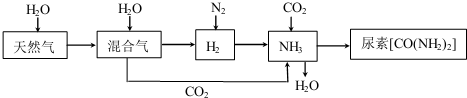
��1����Ȼ���ڸ��¡�������������ˮ������Ӧ���� H2�� CO �Ļ�ѧ��ѧ����ʽΪCH4+H2O$\frac{\underline{\;����\;}}{\;}$CO+3H2��
��2����ͼΪ�ϳɰ���Ӧ�ڲ�ͬ�¶Ⱥ�ѹǿ��ʹ����ͬ���������£���ʼʱ�����������������Ϊ1��3ʱ��ƽ�������а������������

�����ֱ��æ� ��NH���ͦ� ��NH����ʾ�ӷ�Ӧ��ʼ����ƽ��״̬A��Bʱ�Ļ�ѧ��Ӧ���ʣ����A��NH3������B ��NH3�������������������=������
������ͬ�¶ȡ���ѹǿ��p1��Ϊp3ʱ���ϳɰ���Ӧ�Ļ�ѧƽ�ⳣ�����䣮����������С�����䡱����
��3��NH3��g�� ��CO2��g�� ����������Ӧ�������أ�������Ӧ�������仯ʾ��ͼ��ͼ2��NH3��g�� ��CO2��g�� ��Ӧ�������ص��Ȼ�ѧ����ʽΪ2NH3��g��+CO2��g���TCO��NH2��2��s��+H2O��l����H=-134 kJ/mol�˹�����ɲ��ü�ӵ绯ѧ������ȥ��л�����е����أ�ԭ����ͼ3��ʾ���������з����ķ�Ӧ����Ϊ2Cl--2e-=Cl2����CO��NH2��2+3Cl2+H2O=N2+CO2+6HCl��
��4�����䰱ʱ������ʹ��ͭ����Ͻ�����Ĺܵ����ţ���Ϊ�ڳ�ʪ�Ļ����У�����ͭ����NH3����ʱ�ܱ������е�O2����������Cu��NH3��42+���÷�Ӧ�����ӷ���ʽΪ2Cu+8NH3+O2+2H2O�T2Cu��NH3��42++4OH-��
���� ��1��������ˮ������Ӧ����һ����̼��������
��2�����¶�Խ��ѹǿԽ��Ӧ����Խ��
�ڻ�ѧƽ�ⳣ��ֻ���¶��йأ�
��3����ͼʾ��֪����һ����2NH3��g��+CO2��g��?H2NCOONH4��l����������泥���H1=-272KJ/mol��
�ڶ�����H2NCOONH4��l��?H2O��l��+H2NCONH2��s����H2=+138KJ/mol��
���ݸ�˹���ɣ�����������ӵõ�NH3��g����CO2��g����Ӧ�������ص��Ȼ�ѧ����ʽΪ2NH3��g��+CO2��g��?H2O��l��+H2NCONH2��s����H=-134kJ•mol-1���������з����ķ�Ӧ����Ϊ��2Cl--2e-=Cl2����CO��NH2��2+3Cl2+H2O=N2+CO2+6HCl��
��4��ͭ����NH3����ʱ�ܱ������е�O2����������Cu��NH3��42+���Դ���д���ӷ�Ӧ��
��� �⣺��1��������ˮ������Ӧ����һ����̼���������÷�ӦΪCH4+H2O$\frac{\underline{\;����\;}}{\;}$CO+3H2���ʴ�Ϊ��CH4+H2O$\frac{\underline{\;����\;}}{\;}$CO+3H2��
��2�����¶�Խ��ѹǿԽ��Ӧ����Խ����ͼ��֪��B��Ӧ���¶ȡ�ѹǿ����Ӧ���ʴʴ�Ϊ������
�ڻ�ѧƽ�ⳣ��ֻ���¶��йأ���Ȼ�¶Ȳ��䣬��ѧƽ�ⳣ��K���䣬�ʴ�Ϊ�����䣻
��3����ͼʾ��֪����һ����2NH3��g��+CO2��g��?H2NCOONH4��l����������泥���H1=-272KJ/mol��
�ڶ�����H2NCOONH4��l��?H2O��l��+H2NCONH2��s����H2=+138KJ/mol��
���ݸ�˹���ɣ�����������ӵõ�NH3��g����CO2��g����Ӧ�������ص��Ȼ�ѧ����ʽΪ2NH3��g��+CO2��g��?H2O��l��+H2NCONH2��s����H=-134kJ•mol-1���������з����ķ�Ӧ����Ϊ��2Cl--2e-=Cl2����CO��NH2��2+3Cl2+H2O=N2+CO2+6HCl��
�ʴ�Ϊ��2NH3��g��+CO2��g���TCO��NH2��2��s��+H2O��l����H=-134 kJ/mol��2Cl--2e-=Cl2����CO��NH2��2+3Cl2+H2O=N2+CO2+6HCl��
��4��ͭ����NH3����ʱ�ܱ������е�O2����������Cu��NH3��42+�������ӷ�ӦΪ2Cu+8NH3+O2+2H2O�T2Cu��NH3��42++4OH-���ʴ�Ϊ��2Cu+8NH3+O2+2H2O�T2Cu��NH3��42++4OH-��
���� ���⿼���Ʊ�ʵ�鷽������ƣ��ۺ��Խ�ǿ����Ŀ�ѶȽϴ��漰��ѧƽ�⼰���㡢���ӷ�Ӧ��������ԭ��Ӧ���Ȼ�ѧ��Ӧ�ȸ߿��������㣬ע��ѧ��������ѵ��������ͼ�����ݴ������ɽ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | HNO3��K2CO3��Ba��OH��2 | B�� | Ba��OH��2��HNO3��K2CO3 | ||
| C�� | K2CO3��Ba��OH��2��HNO3 | D�� | Ba��OH��2��K2CO3��HNO3 |
| A�� | ����ˮ���γɵ�Al��OH��3����������ˮ�������������ˮ�ľ��� | |
| B�� | �ں������������п�飬�ɼ�������ĸ�ʴ���� | |
| C�� | �����Ƿ���ж����ЧӦ������ɢϵ��Ϊ��Һ����Һ������ | |
| D�� | ��ҵ�ϵ�����ڵ�MgCl2�����Ƶý���þ |
| A�� | pH��ȵĢ�NH4Cl �ڣ�NH4��2SO4 ��NH4HSO4��Һ��c��NH4+����С˳��Ϊ�٣��ڣ��� | |
| B�� | 0��l mol•L-1CuSO4•��NH4��2SO4•6H2O��Һ��c��SO42-����c��NH4+����c��Cu2+����c��OH-����c��H+�� | |
| C�� | 0.1 mol•L-1Na2CO3��Һ��C��Na+��+c��H+��=c��HCO3-��+c��CO32-��+c��OH-�� | |
| D�� | ������������ʵ���Ũ�ȵ�CH3COONa��CH3COOH��Ϻ����Һ��c��CH3COO-��+2c��OH-��=2c��H+��+c��CH3COOH�� |
| A�� | ����ͨʽ��CnH2n-2���л��������Ȳ���������Ƕ�ϩ���ͻ�ϩ�� | |
| B�� | ��ϩ����ʹ����KmnO4��Һ��ɫ������ʹ��ˮ��ɫ������ɫ�ķ�Ӧԭ����ͬ | |
| C�� | ij��������ֻ��һ�������Ҳ���ȫ������������������DZ���ͬϵ�� | |
| D�� | 2-��Ȳ�����е��ĸ�̼ԭ��һ����ͬһ��ֱ���� |
 ��
��