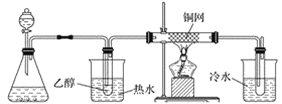
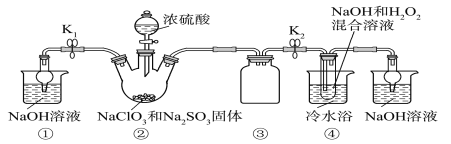
��Ŀ����
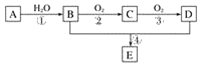
����Ŀ�������۵���ߵĽ���������Ҫ��ս�����ʡ���Ȼ��������Ҫ�����ں��ٿ��У�����Ҫ�ɷ��������̵������Σ�FeWO4��MnWO4������������Si��As�Ļ�����ɺ��ٿ�ұ���ٵĹ����������£�

��֪��
������I����Ҫ�ɷ���Fe2O3��MnO2��
�����������У��ٵĻ��ϼ�ֻ�������һ�������ı䡣
������������������ˮ��
��1����д��FeWO4�����������·�����ֽⷴӦ����Fe2O3�Ļ�ѧ����ʽ��______________________________________��
��2�����������������������Һ�м������к���pH=10����Һ�е�����������ΪSiO32�D��HAsO32�D��HAsO42�D�ȣ����������������У�����H2O2ʱ������Ӧ�����ӷ���ʽΪ_________������������Ҫ�ɷ���______________��
��3����֪�������ƺ�����ƣ�CaWO4�������ܵ���ʣ����ߵ��ܽ�Ⱦ����¶����߶���С����ͼΪ��ͬ�¶���Ca��OH��2��CaWO4�ij����ܽ�ƽ��������

��T1_____T2������>������<����T1ʱKsp��CaWO4��=______________��
������������Һ����ʯ����õ���������ƣ�������Ӧ�����ӷ���ʽΪ__________________________��
���𰸡�4FeWO4+O2+8NaOH![]() 2Fe2O3+4Na2WO4+4H2O H2O2+HAsO32�D�THAsO42�D+H2O MgSiO3 �� MgHAsO4 < 1��10-10 WO42�D+Ca(OH)2=CaWO4+2OH�D
2Fe2O3+4Na2WO4+4H2O H2O2+HAsO32�D�THAsO42�D+H2O MgSiO3 �� MgHAsO4 < 1��10-10 WO42�D+Ca(OH)2=CaWO4+2OH�D
��������
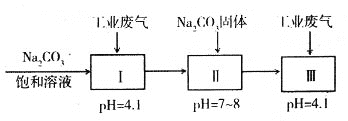
�����̿�֪�������������������ơ�������Ӧ�����������������ƣ������̺��������Ʒ�Ӧ���������ƺ��������̣�ˮ��ʱ�����������������̲�����ˮ������������ˮ���ʹ��˺�õ�����Һ�������ƣ�����I����Ҫ�ɷ���Fe2O3��MnO2�������ƺ�Ũ���ᷴӦ��������������ƣ�����������⣬����+5�۵���Ϊ+6�ۣ������Ȼ�þ������������ˮ��MgSiO3��MgHAsO4�����ˣ���ҺΪ�����ƣ��ữ�����ȷֽ�����������ٺ�ˮ���û�ԭ����ԭ�������������٣��ݴ˷������
��1����������ͼ��֪�����������������ơ�������Ӧ�����������������ƣ���Ӧ�ķ���ʽΪ4FeWO4+O2+8NaOH![]() 2Fe2O3+4Na2WO4+4H2O��
2Fe2O3+4Na2WO4+4H2O��
��2���������Ϸ���������H2O2��Ŀ���ǽ�HAsO32-������HAsO42-�����ӷ���ʽΪH2O2+HAsO32-�THAsO42-+H2O����ҺI�д���SiO32-��HAsO32-��HAsO42-�����ӣ���������pHֵ�����Ȼ�þ��Mg2+����SiO32-��HAsO32-��HAsO42-�����ӣ����������Ҫ�ɷ���MgSiO3��MgHAsO4��
��3���ٸ���ͼ���֪���������ƺ�������ڸ�����Ũ����ͬʱ��T1�¶���������Ũ�ȴ���T2��˵��T1ʱ���ܶȻ�����T2���ܶȻ�Խ�����ܽ��Խ������T1ʱ�ܽ�Ƚϴ����ڡ���֪�������ƺ�����ƣ�CaWO4�������ܵ���ʣ����ߵ��ܽ�Ⱦ����¶����߶���С������T1��T2��T1ʱKsp��CaWO4��=c��Ca2+��c��WO42-��=1��10-5��1��10-5=1��10-10��
�ڽ���������Һ����ʯ���飬�������ֽⷴӦ���������ƺ���������ӷ�Ӧ��������Ƴ�������Ӧ�����ӷ���ʽΪWO42�D+Ca(OH)2=CaWO4+2OH�D��

����Ŀ������Na��Mg��Al�й㷺��Ӧ�á�
��1��Al��Ԫ�����ڱ��е�λ����__________________��
��2������þ�����������������ˣ�Ԫ�ط�����U����UF4+2Mg![]() U+2MgF2���÷�Ӧ�У���Ϊ��ԭ����������_________���ѧʽ����ͬ��������ԭ��������_________��
U+2MgF2���÷�Ӧ�У���Ϊ��ԭ����������_________���ѧʽ����ͬ��������ԭ��������_________��
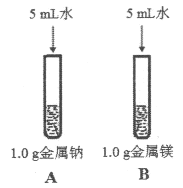
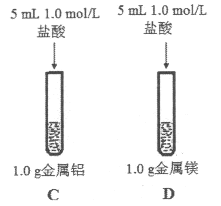
��3��Ϊ�Ƚ�Na��Mg��Al�Ľ����ԣ�����������ʵ�飨��������ı��������ͬ����
ʵ��1 | ʵ��2 |
|
|
����ˮ��Ӧ���ң�þ��ˮ��Ӧ���� | þ�����ᷴӦ���ң��������ᷴӦ���� |
��ʵ��1��ʵ��2�ó��Ľ����ǣ�������_________>_________>_________����Ԫ�ط��ţ�����ԭ�ӽṹ���۽��ͣ�ͬ����Ԫ�ش����ң�_________��
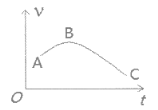
��4����þ����ȥ������Ĥ��Ͷ�뵽ʢ������ij��������У�����H2������v��ʱ��t�Ĺ�ϵ��ͼ��ʾ��AB�������������Ҫԭ����__________________��