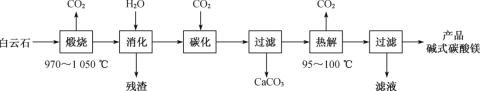
��Ŀ����
����Ŀ����ҵ����CO2��NH3Ϊԭ�Ϻϳ����ء������غϳ����е���Ҫ��Ӧ�ɱ�ʾ���£�
��Ӧ��2NH3(g)��CO2(g)![]() NH2CO2NH4(s)����H1����159.47 kJ��mol��1
NH2CO2NH4(s)����H1����159.47 kJ��mol��1
��Ӧ��NH2CO2NH4(s) ![]() CO(NH2)2(s)��H2O(g)����H2����72.49 kJ��mol��1
CO(NH2)2(s)��H2O(g)����H2����72.49 kJ��mol��1
�ܷ�Ӧ��2NH3(g)��CO2(g) ![]() CO(NH2)2(s)��H2O(g)����H3
CO(NH2)2(s)��H2O(g)����H3
�ش��������⣺
(1)����ͬ�����£���Ӧ��ֱ��ں��º��������к;��Ⱥ��������н��У����߾��ﵽƽ���c(CO2)����________c(CO2)����(������������С��������������)����H3��____________��
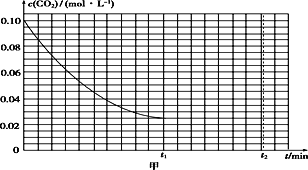
(2)ij�о�С��Ϊ̽����Ӧ����Ӱ��c(CO2)�����أ��ں����½�0.4 mol NH3��0.2 mol CO2�����ݻ�Ϊ2 L���ܱ������У�t1ʱ�ﵽ��ѧƽ�⣬c(CO2)��ʱ��t�仯������ͼ����ʾ����0��t1ʱ���ڸû�ѧ��Ӧ������(NH3)��____________���������������䣬t1ʱ���������ѹ����1 L��t2ʱ�ﵽ�µ�ƽ�⡣����ͼ���л���t1��t2ʱ����c(CO2)��ʱ��t�仯�����ߡ�___________________________________
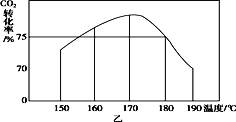
(3)��150 ��ʱ����2 mol NH3��1 mol CO2����a L �ܱ������У�������Ӧ����tʱ�̣����������CO2ת����ԼΪ73%��Ȼ��ֱ����¶�Ϊ160 �桢170 �桢180 �桢190 ��ʱ������������ʼʵ���������䣬�ظ�����ʵ�飬������ͬʱ����CO2ת���ʲ����Ʊ仯����(��ͼ��)����150��170 ��֮�䣬CO2ת���ʳ���������ı仯���ƣ���ԭ����__________180 ��ʱ��Ӧ���ƽ�ⳣ��K��____________(�ú�a��ʽ�ӱ�ʾ)��
(4)�����Ƽ��Ҫԭ��ΪNaCl��CO2��NH3������Ҫ����ƷΪNH4Cl����֪�����£�NH3��H2O�ĵ��볣��Kb��1.8��10��5����0.2 mol��L��1 NH4Cl��Һ��pHԼΪ________(ȡ��������)��
���𰸡�С�� ��86.98 kJ��mol��1 ![]() molL-1min-1
molL-1min-1 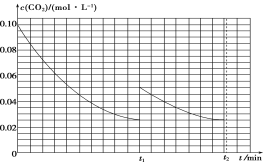 ��Ӧδ�ﵽƽ��״̬���¶�Խ�߷�Ӧ����Խ�죬CO2ת����Խ��ƽ�⿿£ 12a2 5
��Ӧδ�ﵽƽ��״̬���¶�Խ�߷�Ӧ����Խ�죬CO2ת����Խ��ƽ�⿿£ 12a2 5
��������
��1����Ӧ��ֱ��ں��º��ݺ;��Ⱥ����н��У����߾��ﵽƽ�⣬�����������¶�����ƽ��������У�������̼Ũ�������ø�˹���ɼ���H3��
��2����ͼ��֪��0��t1ʱ���ڣ�������̼Ũ�ȵı仯��Ϊ0.1mol/L-0.025mol/L=0.075mol/L��������=![]() ���м��㣻���ѹ��Ϊԭ����һ�룬Ũ��˲ʱ����Ϊԭ����һ������Ϊ0.05mol/L������ѹǿƽ�������ƶ�����������Ϊ���壬���մﵽ��ƽ��״̬ʱCO2��Ũ����Ϊ0.025mol/L���ݴ˻������ߣ�
���м��㣻���ѹ��Ϊԭ����һ�룬Ũ��˲ʱ����Ϊԭ����һ������Ϊ0.05mol/L������ѹǿƽ�������ƶ�����������Ϊ���壬���մﵽ��ƽ��״̬ʱCO2��Ũ����Ϊ0.025mol/L���ݴ˻������ߣ�
��3����ͼ�ҿ�֪������̼��ת�������¶ȵ����߶������¶ȴﵽ170��ʱ�ﵽ���ֵ����Ӧ�ﵽƽ��״̬���¶ȼ������ߣ�������̼��ת������С��˵��ƽ��������У�180 ��ʱ������̼��ת����Ϊ75%���������η����м��㣻
��4��笠�����ˮ��ʹ��Һ�����ԣ�NH4+��ˮ��ƽ�ⳣ��Kh=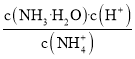 =
=![]() ��c2(H+)=Kh��c(NH4+)�����c(H+)����һ������pH��
��c2(H+)=Kh��c(NH4+)�����c(H+)����һ������pH��
��1����Ӧ��ֱ��ں��º��ݺ;��Ⱥ����н��У����߾��ﵽƽ�⣬���ڷ�ӦI�Ƿ��ȷ�Ӧ�������������¶�����ƽ��������У�������̼Ũ������c(CO2)����С��c(CO2)��Ե�����ø�˹���ɿ�֪I+II���ܷ�Ӧ��2NH3(g)��CO2(g)![]() CO(NH2)2(s)��H2O(g)����H3=-86.98 kJ��mol��1��
CO(NH2)2(s)��H2O(g)����H3=-86.98 kJ��mol��1��
��2����ͼ��֪��0��t1ʱ���ڣ�������̼Ũ�ȵı仯��Ϊ0.1mol/L-0.025mol/L=0.075mol/L����c��NH3��=0.15mol/L�� ��(NH3)=![]() =
=![]() =
=![]() molL-1min-1�����ѹ��Ϊԭ����һ�룬Ũ��˲ʱ����Ϊԭ����һ������Ϊ0.05mol/L������ѹǿƽ�������ƶ�����������Ϊ���壬���մﵽ��ƽ��״̬ʱCO2��Ũ����Ϊ0.025mol/L���ݴ�����Ϊ��
molL-1min-1�����ѹ��Ϊԭ����һ�룬Ũ��˲ʱ����Ϊԭ����һ������Ϊ0.05mol/L������ѹǿƽ�������ƶ�����������Ϊ���壬���մﵽ��ƽ��״̬ʱCO2��Ũ����Ϊ0.025mol/L���ݴ�����Ϊ��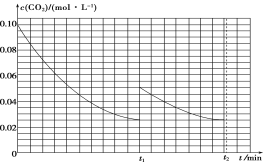 ��
��
��3����ͼ�ҿ�֪������̼��ת�������¶ȵ����߶������¶ȴﵽ170��ʱ�ﵽ���ֵ����Ӧ�ﵽƽ��״̬���¶ȼ������ߣ�������̼��ת������С��˵��ƽ��������У�������150��170 ��֮�䣬CO2ת���ʳ���������ı仯���ƣ���ԭ���Ƿ�Ӧδ�ﵽƽ��״̬���¶�Խ�߷�Ӧ����Խ�죬CO2ת����Խ��ƽ�⿿£��
��CO2ת����x mol/L
2NH3(g)��CO2(g)![]() CO(NH2)2(s)��H2O(g)
CO(NH2)2(s)��H2O(g)
ʼ��mol/L�� ![]()
![]() 0
0
ת��mol/L�� 2x x x
ƽ��mol/L�� ![]() -2x
-2x ![]() -x x
-x x
180 ��ʱ������̼��ת����Ϊ75%������![]() 100%=75%�����x=
100%=75%�����x=![]()
ƽ�ⳣ��K��![]() =
=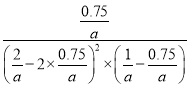 =12a2��
=12a2��
��4��笠�����ˮ��ʹ��Һ�����ԣ�NH4��ˮ��ƽ�ⳣ��Kh=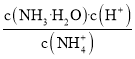 =
=![]() =
=![]() ��ˮ�����ɵ�c(NH3��H2O)���Ƶ���c(H+)����c2(H+)=Kh��c(NH4+)��c(H+)=
��ˮ�����ɵ�c(NH3��H2O)���Ƶ���c(H+)����c2(H+)=Kh��c(NH4+)��c(H+)=![]() =
=![]() ��10-5mol/L��pH=-lg[c(H+)]=5��
��10-5mol/L��pH=-lg[c(H+)]=5��