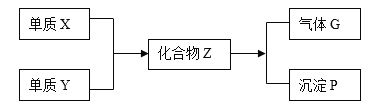
��Ŀ����
����Ŀ���� NA Ϊ�����ӵ���������ֵ������˵����ȷ����
A.�����£�5.6g ����Ͷ��������Ũ�����У�ת�Ƶ�����Ϊ 0.3NA
B.0.5mol ��������(![]() )�к��еĹ��ۼ���ĿΪ 1.5NA
)�к��еĹ��ۼ���ĿΪ 1.5NA
C.2.4g þ����������N2��O2�Ļ����������ȫȼ�գ�ת�Ƶ�����Ϊ 0.2NA
D.25��ʱ��Ksp(BaSO4)=1��10-10����BaSO4������Һ��Ba2+��ĿΪ1��10-5NA
���𰸡�C
��������
A�������£�����Ũ���ᷢ���ۻ�����ֹ�˷�Ӧ�Ľ��У���������ȫ��Ӧ�����ܼ���ת�Ƶĵ���������A����
B�����������к�4��C-H����1��C-C����2��C-O��������7�����ۼ�����0.5mol���������к��еĹ��ۼ���ĿΪ3.5NA����B����
C��2.4gþ�����ʵ���Ϊ0.1mol����þ��Ӧ���Ϊ+2�ۣ���0.1molþת��0.2NA�����ӣ���C��ȷ��
D������n=cV��֪����Һ���δ֪�������㱵������Ŀ����D����
��ѡC��

 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�����Ŀ����10 L�����ܱ������г���X(g)��Y(g)��������ӦX(g)��Y(g) ![]() M(g)��N(g)������ʵ���������±���
M(g)��N(g)������ʵ���������±���
ʵ�� ��� | �¶�/�� | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | ||
n(X) | n(Y) | n(M) | |||
�� | 700 | 0.40 | 0.10 | 0.090 | |
�� | 800 | 0.10 | 0.40 | 0.080 | |
�� | 800 | 0.20 | 0.30 | a | |
�� | 900 | 0.10 | 0.15 | b | |
����˵����ȷ����(����)
A. ʵ�����У���5 minʱ���n(M)��0.050 mol����0��5 minʱ���ڣ���N��ʾ��ƽ����Ӧ����v(N)��1.0��10��2 mol��L��1��min��1
B. ʵ�����У��÷�Ӧ��ƽ�ⳣ��K��2.0
C. ʵ�����У��ﵽƽ��ʱ��X��ת����Ϊ60%
D. ʵ�����У��ﵽƽ��ʱ��b>0.060
����Ŀ��ij�жԴ������м�⣬���ָ�����Ҫ��Ⱦ��Ϊ�����������PM2.5��ֱ��С�ڵ���2.5��m���������������Ҫ��ԴΪȼú��������β���ȡ���ˣ���PM2.5��SO2��NOx�Ƚ����о�������Ҫ���塣
(1)��PM2.5����������ˮ�����Ƴɴ�������������ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�������
���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
Ũ��/molL-1 | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
���ݱ��������ж�PM2.5�������Ϊ_______��������pH=_______��
(2)Ϊ����SO2���ŷţ�����ȡ�Ĵ�ʩ�ǽ�úת��Ϊ�������ȼ�ϡ���֪��
H2(g)+![]() O2(g)=H2O(g) ��H=-241.8kJ��mol-1
O2(g)=H2O(g) ��H=-241.8kJ��mol-1
C(s)+![]() O2(g)=CO(g)��H=-110.5kJ��mol-1
O2(g)=CO(g)��H=-110.5kJ��mol-1
��C(s)+H2O(g)=CO(g)+H2(g)����H=________kJ��mol-1��
(3)����β����NOx��CO�����ɼ�ת��Ϊ��
����֪����������NO�ķ�ӦΪ��N2(g)+O2(g)![]() 2NO(g) ��H��0����1mol��������0.8molN2��0.2molO2��1300��ʱ���ܱ������ڷ�Ӧ�ﵽƽ�⡣���NOΪ8��10-4mol��������¶��µ�ƽ�ⳣ��K=_______�����������������¶�Խ�ߣ���λʱ����NO�ŷ���Խ��ԭ����________��
2NO(g) ��H��0����1mol��������0.8molN2��0.2molO2��1300��ʱ���ܱ������ڷ�Ӧ�ﵽƽ�⡣���NOΪ8��10-4mol��������¶��µ�ƽ�ⳣ��K=_______�����������������¶�Խ�ߣ���λʱ����NO�ŷ���Խ��ԭ����________��
������ȼ�Ͳ���ȫȼ��ʱ����CO���������밴���з�Ӧ��ȥCO��2CO(g)=2C(s)+O2(g)����֪�÷�Ӧ����H��0�������������ܷ�ʵ�ֵ����ݣ�_________��