��Ŀ����
����Ŀ��ij�жԴ������м�⣬���ָ�����Ҫ��Ⱦ��Ϊ�����������PM2.5��ֱ��С�ڵ���2.5��m���������������Ҫ��ԴΪȼú��������β���ȡ���ˣ���PM2.5��SO2��NOx�Ƚ����о�������Ҫ���塣
(1)��PM2.5����������ˮ�����Ƴɴ�������������ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�������
���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
Ũ��/molL-1 | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
���ݱ��������ж�PM2.5�������Ϊ_______��������pH=_______��
(2)Ϊ����SO2���ŷţ�����ȡ�Ĵ�ʩ�ǽ�úת��Ϊ�������ȼ�ϡ���֪��
H2(g)+![]() O2(g)=H2O(g) ��H=-241.8kJ��mol-1
O2(g)=H2O(g) ��H=-241.8kJ��mol-1
C(s)+![]() O2(g)=CO(g)��H=-110.5kJ��mol-1
O2(g)=CO(g)��H=-110.5kJ��mol-1
��C(s)+H2O(g)=CO(g)+H2(g)����H=________kJ��mol-1��
(3)����β����NOx��CO�����ɼ�ת��Ϊ��
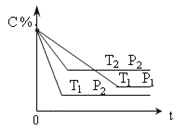
����֪����������NO�ķ�ӦΪ��N2(g)+O2(g)![]() 2NO(g) ��H��0����1mol��������0.8molN2��0.2molO2��1300��ʱ���ܱ������ڷ�Ӧ�ﵽƽ�⡣���NOΪ8��10-4mol��������¶��µ�ƽ�ⳣ��K=_______�����������������¶�Խ�ߣ���λʱ����NO�ŷ���Խ��ԭ����________��
2NO(g) ��H��0����1mol��������0.8molN2��0.2molO2��1300��ʱ���ܱ������ڷ�Ӧ�ﵽƽ�⡣���NOΪ8��10-4mol��������¶��µ�ƽ�ⳣ��K=_______�����������������¶�Խ�ߣ���λʱ����NO�ŷ���Խ��ԭ����________��
������ȼ�Ͳ���ȫȼ��ʱ����CO���������밴���з�Ӧ��ȥCO��2CO(g)=2C(s)+O2(g)����֪�÷�Ӧ����H��0�������������ܷ�ʵ�ֵ����ݣ�_________��
���𰸡����� 4 +131.3 4��10-6 �¶����ߣ���Ӧ���ʼӿ죬ƽ�������ƶ� �ؼ������ķ�Ӧ���κ��¶��²����Է�����
��������
(1)�۲�����з���NH4+ˮ�������ԣ�PM2.5�������Ϊ���ԣ�
(2)���ø�˹���ɼ��㷴Ӧ�ȣ�
(3)�ټ����ƽ��ʱ�������ʵ����ʵ���Ũ�ȣ����ƽ�ⳣ���ı���ʽ���㣻���ݻ�ѧ��Ӧ���ʺ�ƽ���ƶ�ԭ�������жϣ�
�ڸ��ݷ�Ӧ�������ܹ�ʽ�����жϡ�
(1)�۲�����з���NH4+ˮ�������ԣ���PM2.5�������Ϊ������Һ��������pHֵ������Һ�е���غ�ɵ�c(K+)+c(Na+)+c(NH4+)+c(H+)=2c(SO42-)+c(NO3-)+c(Cl-)�����������ݴ����ʽ�ӣ��ɵ�4��10-6+6��10-6+2��10-5+c(H+)=2��4��10-5+3��10-5+2��10-5�����c(H+)=10-4mol/L�����Ը���Һ��pH=4��
(2)����֪����H2(g)+![]() O2(g)=H2O(g) ��H=-241.81kJmol-1
O2(g)=H2O(g) ��H=-241.81kJmol-1
��C(s)+![]() O2(g)=CO(g)��H=-110.5kJ��mol-1
O2(g)=CO(g)��H=-110.5kJ��mol-1
���ø�˹���ɣ�����-�ٿɵ�C(s)+H2O(g)=CO(g)+ H2(g)��H=(-110.51kJmol-1)-(-241.81kJmol-1)=+13l.3kJmol-1��
(3)����֪����������NO�ķ�ӦΪ��N2(g)+O2(g)![]() 2NO(g) ��H��0���������н���1mol����(1mol��������0.8mol N2��0.2molO2)��1300��ʱ���ܱ������ڷ�Ӧ�ﵽƽ�⣬���NOΪ8��10-4mol����Ӧǰ���������ʵ�����ͬ������ƽ�ⳣ��ʱ���������ʵ�������ƽ��Ũ�ȼ��㣬�ȼ������ʵ�ƽ������n(N)2=0.8mol-4��10-4mol��n(O2)=0.2mol-4��10-4 mol������ƽ�ⳣ������ʽ���ɣ���K=
2NO(g) ��H��0���������н���1mol����(1mol��������0.8mol N2��0.2molO2)��1300��ʱ���ܱ������ڷ�Ӧ�ﵽƽ�⣬���NOΪ8��10-4mol����Ӧǰ���������ʵ�����ͬ������ƽ�ⳣ��ʱ���������ʵ�������ƽ��Ũ�ȼ��㣬�ȼ������ʵ�ƽ������n(N)2=0.8mol-4��10-4mol��n(O2)=0.2mol-4��10-4 mol������ƽ�ⳣ������ʽ���ɣ���K=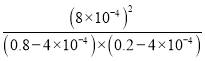 =4��10-6�������¶�Խ�ߣ���λʱ����NO�ŷ���Խ��ԭ�����¶����ߣ���Ӧ���ʼӿ죬��ѧƽ�������ȵ�����Ӧ�����ƶ������������NO���壻
=4��10-6�������¶�Խ�ߣ���λʱ����NO�ŷ���Խ��ԭ�����¶����ߣ���Ӧ���ʼӿ죬��ѧƽ�������ȵ�����Ӧ�����ƶ������������NO���壻
��2CO(g)=2C(s)+O2(g)����֪�÷�Ӧ����H��0�����ݷ���ʽ��֪���÷�Ӧ������Ӧ�����������С�ķ�Ӧ����S��0������ϵ����������G=��H-T��S��0�����Ը÷�Ӧ���ؼ��������ķ�Ӧ�����κ��¶��²����Է����С�

����Ŀ���±��������ʣ��������ǵ���Һ��ͨ��һ����Ӧ��ʵ����ͼ��ʾ��ת������
ѡ�� | X | Y | Z |
|
A | Si | Na2SiO3 | H2SiO3 | |
B | S | H2S | SO2 | |
C | Al2O3 | NaAlO2 | Al2(SO4)3 | |
D | Mg(OH)2 | MgCO3 | MgCl2 |
A.AB.BC.CD.D
����Ŀ��(1)���ʹ�õ��ƽ���ȼ����N��H����Ԫ����ɣ���ԭ�Ӹ���N��H=1��2����ˮ��Һ�Լ��ԣ����������Nԭ�ӵ��ӻ���ʽΪ______________________��
(2)Ц��(N2O)������������������������ʳ�ᵼ������������ҡ�Ԥ��N2O�ĽṹʽΪ________________________��
(3)Ԫ�صĻ�̬��̬ԭ�ӵõ�һ�������γ���̬��1������ʱ���ų�������������һ��������(E)����1���������ٻ��һ�����ӵ������仯�����ڶ��������ܣ�����Ԫ�ػ����ӵĵ��������������±���ʾ��
Ԫ�� | C1 | Br | I | O | O- |
�������ܣ�kJ��mol�� | 349 | 343 | 295 | 141 | ��780 |
����˵����ȷ����___________��
A����������Խ��˵��Խ�ѵõ�����
B��һ����̬����̬��ԭ�ӵõ�һ�����ӳ�ΪO2-ʱ�ų�141kJ������
C����Ԫ�صĵڶ����������ǣ�780kJ��mol
D����̬����̬��ԭ�ӵõ��������ӳ�ΪO2-��Ҫ��������
(4)�ڵ�����������м������ʯ(����A������)��������Al2O3�۵�����á�����ʯ������ԭ��Ϊ��2Al(OH)3+12HF+3Na2CO3=2A+3CO2��+9H2O�������������������գ�
�ٱ���ʯ�Ļ�ѧʽΪ____________________________��
�ڱ���ʯ�����������ɣ�����ʯ�ľ����ṹ��ͼ����ʾ����λ�ڴ�������Ķ�������ģ���λ�ڴ��������12������е��8��С����������ģ���ô������������Ĵ�������������___________(��������)��
�۱���ʯ��Һ�в����ڵ�������������________________(��ѡ����ĸ)��
A ���Ӽ� B ���ۼ� C ��λ�� D ������ E ���»��� F ���
��Al���ʵľ�����ԭ�ӵĶѻ���ʽ��ͼ����ʾ���侧��������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ��ͼ����ʾ��

����֪A1��ԭ�Ӱ뾶Ϊd cm��NA���������ӵ�������Al�����ԭ������ΪM������Alԭ�ӵ���λ��Ϊ________��Al������ܶ�Ϊ__________g��cm-3(����ĸ��ʾ)��
(5)�����Fe(CO)5���۵㣭20�棬�е�103�棬�������Ʊ�������Fe(CO)5�Ľṹ��ͼ��ʾ��

��Fe(CO)5������������__________���塣
�ڹ���Fe(CO)5������˵����ȷ����_____��
A��Fe(CO)5�ǷǼ��Է��ӣ�CO�Ǽ��Է���
B��Fe(CO)5��Feԭ����sp3�ӻ���ʽ��CO�ɼ�
C��1mol Fe(CO)5����10mol���
D����ӦFe(CO)5=Fe+5COû���»�ѧ������