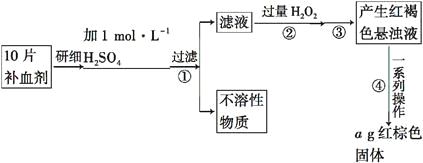
��Ŀ����
����Ŀ����������(Na2SeO3)Ϊ��ɫ���壬 �ڿ������ȶ�������ˮ�����ڴ�����ͼ�������������Ʊ��������Ƶ�����������ش����������

(1)��������ͬ���������Ԫ�أ��������������Ļ��ϼ�Ϊ_________�������ڱ��е�λ����_________��
(2)������м����������ƺϳ�����������Һ������pHֵΪ7~14��pHֵ����7~ 14��ԭ����____________��
(3)�����ٵ�������_______������ͼ����ո������������_____________��
(4)������Ϊ_____________���ᾧ�����ˡ�ϴ�ӣ�Ȼ�������Ļ�˦���ᾧ��Һ���ô��нᾧˮ���������ơ���������ϴ�Ӽ������______(�����)��
a��ϡ���� b����������ϡ��Һ c��ˮ d���Ҵ�
(5)���ڳ����Ե�����Ϊԭ�ϣ�����������ˮ��Һ��ͨ���ѹ�����ķ���������������ơ�д���Ʊ��������ƵĻ�ѧ��Ӧ����ʽ��____________________��
(6)��֪�������ϳ�·���д�����ת��Ϊ��������ʱ��ʧ2%��������ж���������������Ϊ95%��������ĺ��Բ��ƣ��Լ�������1t����79%�Ĵ������տ�����______ t(��ȷ��0.1t)����������95%�IJ�Ʒ��
���𰸡�+4 �������ڵ�VIA ��ֹ��������ˮ�⣬��߲��� ���� ��ˮ ����Ũ�� d Se+O2+2NaOH=Na2SeO3+H2O 1.7
��������
������ͨ�������õ�������������ȥ��ĵ����������ڽ�����м�������������Һ�������˵õ��������������ƣ����������Ƶ���Һ��������Ũ�����ᾧ�����ˡ�ϴ�ӵõ��������Ƶľ��壬����ո��������ˮ�õ��������ƣ��ݴ˷�����
(1)��������(Na2SeO3)������Ԫ�ػ��ϼ�֮�͵���0�����Ļ��ϼ�Ϊ+4���������ڱ���λ���ǵ������ڵ�VIA����������ͬ���������Ԫ�أ������ڱ��е�λ���ǵ������ڵ�VIA��
(2)����������Һ�������ǿ���γɵ��Σ�����ˮ�⣬��Һ�Լ��ԣ���ֹ��������ˮ�⣬pHֵ����7~ 14����߲�����
(3)����������Һ����ķ���Ϊ���ˣ��ʲ����ٵ������ǹ��ˣ�����ͼ����ո��������������ˮ��
(4)������Ϊ����Ũ�����ᾧ�����ˡ�ϴ�ӣ�Ȼ�������Ļ�˦���ᾧ��Һ���ô��нᾧˮ���������ơ���������ϴ�Ӽ�ʱ��ϡ�������������ϡ��Һ�������µ����ʣ�������������ˮ�����ڴ�����ˮ���ܽ���������ƣ�ʹ���ʼ��ͣ��������Ʋ����ڴ������Ҵ��ӷ����׳�ȥ��ѡ���Ҵ�����ʣ�
(5)���ڳ����Ե�����Ϊԭ�ϣ�����������ˮ��Һ��ͨ���ѹ�����ķ���������������ƣ����ǻ�ԭ�������ϼ۴�0�����ߵ�+4�ۣ������������������ϼ۴�0�۽��͵�-2�ۣ��ʲ�����ˮ���ɣ��Ʊ��������ƵĻ�ѧ��Ӧ����ʽSe+O2+2NaOH=Na2SeO3+H2O��
(6)�ڹ��������У�������Ԫ���غ㣬��õ����������Ƶ�����Ϊx���ҵ���ϵʽ��
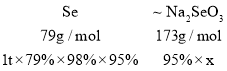
![]() ���õ�x=1.7t��
���õ�x=1.7t��
�ʵõ������������Ϊ1.7t��

 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д�����Ŀ��Ϊ�˺������û�ѧ�ܣ�ȷ����ȫ���������������Ҫ��ֿ��ǻ�ѧ��Ӧ�ķ�Ӧ�ȣ�����ȡ��Ӧ��ʩ����ѧ��Ӧ�ķ�Ӧ��ͨ����ʵ����вⶨ��Ҳ�ɽ����������㡣
��1��ʵ���ã�5g�״���CH3OH��Һ���������г��ȼ�����ɶ�����̼�����Һ̬ˮʱ�ͷų�113.5kJ�����������ʾ�״���ȼ���ȵ��Ȼ�ѧ����ʽΪ�� ��
��2���������������Ȼ�ѧ����ʽ����a b����������������������������
H2(g)+ 1/2O2(g)��H2O(g) ��H1��a kJ��mol-1
H2(g)+ 1/2O2(g)��H2O(l) ��H2��b kJ��mol-1
��3����1mol��̬������ij�ֹ��ۼ���Ҫ���յ������м��ܡ��ӻ�ѧ���ĽǶȷ�������ѧ��Ӧ�Ĺ��̾��Ƿ�Ӧ��Ļ�ѧ�����ƻ���������Ļ�ѧ�����γɹ��̡��ڻ�ѧ��Ӧ�����У���ѧ����Ҫ�����������γɻ�ѧ���ֻ��ͷ�������
��ѧ�� | H��H | N��H | N��N |
����/kJ��mol��1 | 436 | 391 | 945 |
��֪��ӦN2(g)��3H2(g)![]() 2NH3(g) ��H��a kJ��mol��1���Ը��ݱ������м������ݹ���a��ֵ��_______________(ע����+����������)��
2NH3(g) ��H��a kJ��mol��1���Ը��ݱ������м������ݹ���a��ֵ��_______________(ע����+����������)��
��4�����ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ�ķ�Ӧ�Ƚ������㡣����ˮú���ϳɶ����ѵ�������Ӧ���£�
�� 2H2(g) + CO(g)![]() CH3OH(g)����H ����90.8 kJ��mol��1
CH3OH(g)����H ����90.8 kJ��mol��1
�� 2CH3OH(g)![]() CH3OCH3(g) + H2O(g)����H����23.5 kJ��mol��1
CH3OCH3(g) + H2O(g)����H����23.5 kJ��mol��1
�� CO(g) + H2O(g)![]() CO2(g) + H2(g)����H����41.3 kJ��mol��1
CO2(g) + H2(g)����H����41.3 kJ��mol��1
�ܷ�Ӧ��3H2(g) + 3CO(g)![]() CH3OCH3(g) + CO2(g)����H�� ��
CH3OCH3(g) + CO2(g)����H�� ��