��Ŀ����
X��Y��Z��Q��T����Ԫ��λ��ǰ�����ڣ���ԭ��������������X��Y�ĵ����ڳ��³�ѹ�³���̬��X �γɵĵ��ʷ������µ��Ӷԣ�Y�Ļ�̬ԭ����p�ܼ���������δ�ɶԵ��ӣ�Zԭ�ӵİ뾶��ͬ��������Ԫ������� Q�����������γ������һ����Ҫ���ʣ�T2+�����еĵ���������Z��Qԭ�ӵĵ�����֮�͡�
��1��QԪ��λ��Ԫ�����ڱ��� ���� �壬T2+�ĵ����Ų�ʽΪ ��
��2��YԪ�غ�QԪ�طֱ��γɵ�������Ƶ��⻯���У��е�ϸߵ��� ��д��ѧʽ����ԭ����
�ö��Ե缫���X��Y��Z����Ԫ���γɵĻ�����ˮ��Һ���������ĵ缫��ӦʽΪ ��
��3��Z��Q�γɵĻ������ˮ��Һ�� ����ԡ��������ԡ����ԡ�����ԭ��
�� �������ӷ���ʽ��ʾ����
��4����֪��25�桢101325Paʱ��
2T(s)+Q(s)=T2Q(s) ��H=-79.5KJ/mol
Q(s)+Y2(g)=QY2(g) ��H=-296.6KJ/mol
����������Ӧ����д��T2Q��Y2��Ӧ����T�� QY2���Ȼ�ѧ����ʽ ��
��1��QԪ��λ��Ԫ�����ڱ��� ���� �壬T2+�ĵ����Ų�ʽΪ ��
��2��YԪ�غ�QԪ�طֱ��γɵ�������Ƶ��⻯���У��е�ϸߵ��� ��д��ѧʽ����ԭ����
�ö��Ե缫���X��Y��Z����Ԫ���γɵĻ�����ˮ��Һ���������ĵ缫��ӦʽΪ ��
��3��Z��Q�γɵĻ������ˮ��Һ�� ����ԡ��������ԡ����ԡ�����ԭ��
�� �������ӷ���ʽ��ʾ����
��4����֪��25�桢101325Paʱ��
2T(s)+Q(s)=T2Q(s) ��H=-79.5KJ/mol
Q(s)+Y2(g)=QY2(g) ��H=-296.6KJ/mol
����������Ӧ����д��T2Q��Y2��Ӧ����T�� QY2���Ȼ�ѧ����ʽ ��
��14�֣�
��1��������A (��1��) 1s22s22p63s23p63d9 (2��)
��2��H2O ˮ���Ӽ������� (2��) 4OH-+4e-="2" H2O+O2�� (2��)
��3������ ��1�֣� S2- + H2O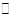 HS-+ OH-��2�֣�
HS-+ OH-��2�֣�
��4��Cu2S��s��+O2��g��=2Cu��s��+ SO2 ��g����H=-217.1KJ/mol ��3�֣�
��1��������A (��1��) 1s22s22p63s23p63d9 (2��)
��2��H2O ˮ���Ӽ������� (2��) 4OH-+4e-="2" H2O+O2�� (2��)
��3������ ��1�֣� S2- + H2O
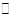 HS-+ OH-��2�֣�
HS-+ OH-��2�֣���4��Cu2S��s��+O2��g��=2Cu��s��+ SO2 ��g����H=-217.1KJ/mol ��3�֣�
���������X��Y�ĵ����ڳ��³�ѹ�³���̬��X �γɵĵ��ʷ������µ��Ӷԣ���X�����е��Ӿ��γɹ��õ��Ӷԣ�ֻ��ΪH��Y�Ļ�̬ԭ����p�ܼ���������δ�ɶԵ��ӣ���֪p�ܼ���2��4�����ӣ�����Ϊ��̬��ֻ��O��Q�����������γ������һ����Ҫ���ʣ�����ΪN��S����Q��ԭ����������Y��ֻ��ΪS��ͬ�����Է�����ZΪNa��T2+�����еĵ���������Z��Qԭ�ӵĵ�����֮�ͣ���֪TΪCu��
��1��Sλ��Ԫ�����ڱ��������ڢ�A��,Cu2+�ĺ�������Ų�ʽΪ1s22s22p63s23p63d9��
��2������ˮ���Ӽ���������ʹ�۷е�H2O> H2S���ö��Ե缫���NaOHˮ��Һ�����ŵ����OH-,
��3��Na2SΪǿ�������Σ���Һ�ʼ��ԣ�ԭ����S2-��ˮ�⣺S2- + H2O
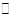 HS-+ OH-��
HS-+ OH-����4���ɢ�ʽ2Cu (s)+S(s)= Cu2S(s) ��H1= -79.5KJ/mol
��ʽS(s)+O2(g)=SO2(g) ��H2= -296.6KJ/mol���ݸ�˹���ɿ��Լ���õ���
��-��=Cu2S��s��+O2��g��=2Cu��s��+ SO2 ��g�� ��H=��H2-��H1=-217.1KJ/mol��

��ϰ��ϵ�д�
 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�
�����Ŀ
 CaCN2��C��CaCN2(�谱����)��ˮ��Ӧ������NH3��
CaCN2��C��CaCN2(�谱����)��ˮ��Ӧ������NH3��