��Ŀ����
17��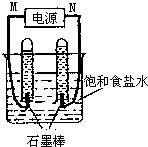 ���÷�ӦCO��g��+H2��g��+O2��g��?H2O��g��+CO2��g����ƶ��ɵ�MCFSȼ�ϵ����һ�����͵�أ����Ը�ȼ�ϵ��Ϊ��Դ����ʯī���缫��ⱥ��NaCl��Һ����Ӧװ�ü�������ͼ��ʾ�����MӦ�ǵ�Դ�ĸ�������������������ڸõ�ⷴӦ�Ļ�ѧ����ʽ��2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+H2��+Cl2��������֪����ʳ��ˮ�����Ϊ1L��һ��ʱ��������Թ����������Ϊ11.2mL����״�����������ǰ����Һ������仯���Բ��ƣ�������Һ��Ͼ��ȣ���ʱ��Һ��pHΪ11��
���÷�ӦCO��g��+H2��g��+O2��g��?H2O��g��+CO2��g����ƶ��ɵ�MCFSȼ�ϵ����һ�����͵�أ����Ը�ȼ�ϵ��Ϊ��Դ����ʯī���缫��ⱥ��NaCl��Һ����Ӧװ�ü�������ͼ��ʾ�����MӦ�ǵ�Դ�ĸ�������������������ڸõ�ⷴӦ�Ļ�ѧ����ʽ��2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+H2��+Cl2��������֪����ʳ��ˮ�����Ϊ1L��һ��ʱ��������Թ����������Ϊ11.2mL����״�����������ǰ����Һ������仯���Բ��ƣ�������Һ��Ͼ��ȣ���ʱ��Һ��pHΪ11��
���� �ö��Ե缫��ⱥ��ʳ��ˮ����������ʧ���ӷ���������Ӧ�������������������ӵõ����ӷ�����ԭ��Ӧ������������Һ��ˮ�ĵ��뱻�ٽ������������ƣ����NaCl�������ٵ��ˮ2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2�����ö��Ե缫��ⱥ��ʳ��ˮʱ�������������ӷŵ磬�����������ӷŵ磻�����������������ƵĹ�ϵʽ�����������Ƶ����ʵ���Ũ�ȣ��Ӷ��ó���Һ��pH��
��� �⣺�ö��Ե缫��ⱥ��ʳ��ˮ������������ʧ���ӷ���������Ӧ�������������������ӵõ����ӷ�����ԭ��Ӧ������������Һ��ˮ�ĵ��뱻�ٽ������������ƣ����NaCl�������ٵ��ˮ2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2��������ͼʾ1��Ӧװ�ü������֪M�����ӵ��Թ����ɵ�����࣬Ӧ�ǵ�Դ�ĸ�����
�ö��Ե缫��ⱥ��ʳ��ˮʱ�������������ӷŵ磬�����������ӷŵ磬ͬʱ��Һ�л������������ƣ����Ե�ط�ӦʽΪ2NaCl+2H2O $\frac{\underline{\;���\;}}{\;}$2NaOH+H2��+Cl2����
�������Թ����������Ϊ11.2mLΪ���������������Ƶ����ʵ���Ũ����xmol/L��
2NaCl+2H2O $\frac{\underline{\;���\;}}{\;}$2NaOH+H2��+Cl2����ת�Ƶĵ��� ���ɵ���������
22.4L 2mol 2mol
0.0112L xmol Xmol
x=$\frac{2mol��0.0112L}{22.4L}$=0.001mol����֪����ʳ��ˮ�����Ϊ1L�����ǰ����Һ������仯���Բ��ƣ���C��H+��=$\frac{K{\;}_{W}}{C��OH{\;}^{-}��}$=$\frac{10{\;}^{-14}}{10{\;}^{-3}}$mol/L=10-11mol/L��PH=-lgc��H+��=11��
�ʴ�Ϊ������2NaCl+2H2O $\frac{\underline{\;���\;}}{\;}$2NaOH+H2��+Cl2���� 11��
���� ���⿼�����ԭ������Һ��pH�����֪ʶ����ȷ�ƶ�ԭ����������ǽⱾ��Ĺؼ����ѵ��Ǽ�����Һ��pH����Ŀ�Ѷ��еȣ�

| A�� | ��ˮ�ܵ��磬˵�������ǵ���� | |
| B�� | ���Ƶ���ˮ�д���3 �ַ��ӣ�4 ������ | |
| C�� | ˫��ˮ����Ϊ����ɫ���������仹ԭ����ΪO2 | |
| D�� | ��ˮ��Ũ�����Ũ���ᰴ�����1��3 ��� |
| A�� | ���ʵ���֬�����ŵ�������ζ����������֬��ˮ������ˮ�ⷴӦ | |
| B�� | ��������Һ��������ԭ������ʯī���缫��ⱥ���Ȼ�����Һ���Ƶ��н�ǿɱ������������Һ | |
| C�� | ������������ڻ���������ҽ�þƾ�������Ƥ����������ԭ������ڿ���ʹ������ϸ�����ڵĵ����ʱ��� | |
| D�� | ��ͥ�в��������Ͻ��������ڴ�Ųˡ�����ʳƷ |
| A�� | ���ᡢ����þ��̼���ơ��������� | B�� | ����ء�̼���ơ��Ȼ��ơ��Ȼ��� | ||
| C�� | ���ᡢ�����ơ�ƫ�����ơ��������� | D�� | ���ᡢ���������Ȼ��ơ��������� |
2NH3��g��+CO2��g��?CO��NH2��2��I��+H2O��I����H��I��
��1����֪�ϳ����صķ�Ӧ���������У�
2NH3��g��+CO2��g��?NH2COONH4��s����H1
NH2COONH4��s��?CO��NH2��2��I��+H2O��I����H2
�������仯������ͼ1��ʾ�����H����H1�͡�H2�ɴ�С��˳��Ϊ��H2����H����H1��
��2����һ����պ����ܱ������г���CO2��NH3������Ӧ��I���ϳ����أ��㶨�¶��»��������NH3�����������ͼ2��ʾ��
A�������Ӧ����v����CO2����B����淴Ӧ����v����CO2�������������������=������CO2��ƽ��ת����Ϊ75%��
��3����һ�����İ�������粒������ں�����������У�������Ӧ��H2NCOONH4��s��?2NH3��g��+CO2��g�����ڲ�ͬ�¶ȣ�T1��T2���£��÷�Ӧ��ƽ��״̬ʱ�������ݼ��±���
| �¶� | ƽ��Ũ��/��mol•L-1�� | |
| c��NH3�� | c��CO2�� | |
| T1 | 0.1 | |
| T2 | 0.1 | |
��������˵���÷ֽⷴӦ�ﵽƽ��״̬����ac������ţ���
a��v������NH3��=2v������CO2��
b���ܱ����������ʵ�����������
c���ܱ������л��������ܶȲ���
d���ܱ������а����������������
��4����������識���ˮ���̼��泥�����������ˮ������ף�25��ʱ����1L 0.1mol•L-1�����������백������立�ĩ����Һ�����ԣ�������Һ����仯��������ȥ0.052mol��������泥���ʱ��Һ�м�������̼Ԫ�أ���ʱ��Һ��c��NH4+��=0.1mol/L��NH4+ˮ��ƽ�ⳣ��ֵΪ4��10-9��

| A�� | ���С� ���İ�װ���Ͽ���������װʳƷ ���İ�װ���Ͽ���������װʳƷ | |
| B�� | �ƾ�����ԭ���ǽ������������� | |
| C�� | GaN�еĵ����ؾ����ڵ�IIIA��Ԫ�� | |
| D�� | ��ѿ�ǵ�ˮ������ܷ���������Ӧ |
| ѡ�� | ʵ��Ŀ�� | ��Ҫ���� | �Լ� |
| A | �ⶨ�к��� | ��Ͳ���¶ȼơ��ƾ��� | ���ᡢNaOH��Һ |
| B | �Ʊ������������� | �ձ�����ͷ�ιܡ��ƾ��� | ����FeCl3��Һ |
| C | ����10%CuSO4��Һ100g | 100ml ����ƿ�������� | ���� |
| D | ʵ������ȡ���ռ�����İ��� | ���Թܡ��ƾ��� | �Ȼ�李���ʯ�ҡ���ˮCaCl2 |
| A�� | A | B�� | B | C�� | C | D�� | D |

