��Ŀ����
����Ŀ��Ϊ�о�ͭ��Ũ����ķ�Ӧ��ij��ѧ��ȤС���������ʵ�顣
ʵ��I����Ӧ����Ķ���̽��,����ͼװ��(�̶�װ������ȥ)����ʵ�飺
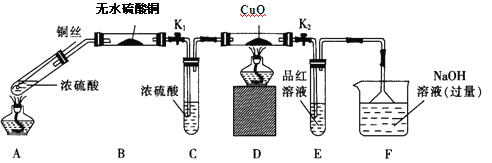
��1��Fװ�õ��ձ��з�����Ӧ�����ӷ���ʽ��_____________��Bװ���е�������__________��
��2��ʵ������У���֤��Ũ��������Ԫ�ص�������ǿ����Ԫ�ص�������_________��
��3��ʵ�����ʱ����ȥ���оƾ���֮ǰ������ɵ�ʵ�������_________��
��4��ʵ�������֤��Aװ���Թ��з�Ӧ���ò����Ƿ���ͭ���ӵIJ���������_________��
ʵ��������Ӧ����Ķ���̽��
��5����ͭ��Ũ���ᷴӦ�Ĺ����У������к�ɫ���ʳ��֣��Һ�ɫ����ΪCu2S������Cu2S�ķ�ӦΪaCu+bH2SO4=cCu2S��dCuSO4��eH2O����a��b=______
��6��Ϊ�ⶨ����ͭ�IJ��ʣ����÷�Ӧ������Һ�кͺ����Ƴ�250��00 mL��Һ��ȡ����Һ25��00 mL�������� KI��Һ�����Ե�����ҺΪָʾ������0.36 mol��L-1��Na2S2O3��Һ�ζ����ɵ�I2��3��ʵ��ƽ�����ĸ�Na2S2O3��Һ25��00 mL������Ӧ����ͭ������Ϊ6��4 g��������ͭ�IJ���Ϊ___��
(��֪![]() )
)
���𰸡�SO2+2OH-=SO32-+H2O ��ˮ����ͭ��ˮ���� Dװ��������ͭ��ɫ�ޱ仯��Eװ����Ʒ����Һ��ɫ ����ͭ˿���ر�K1��K2 ��Aװ������ȴ�Ļ����Һ���ձ��ڱڻ�������ʢˮ���ձ��У������Ͻ��裬�۲��Ƿ���� 5:4 90%
��������
��1��F����NaOH��Һ���ն����SO2���巢�������ӷ�Ӧ����ʽΪSO2+2OH-=SO32-+H2O��B����ˮ����ͭ��ˮ������
��2������A�з�Ӧ��������з��������D���к�ɫ�仯Ϊ��ɫ������֤��A���������ɣ�ͬ��E��Ʒ����ɫ֤��A�����ɶ���������ôDװ��������ͭ��ɫ���仯��Eװ����Ʒ����Һ��ɫ�����˵�������˶������������û����������֤��Ũ��������Ԫ�ص�������ǿ����Ԫ�ء�
��3��ʵ�����ʱ����ȥ���оƾ���֮ǰΪ��ֹ��������SO2������ͭ˿��Ϊ�������ر�K1��K2 ��
��4����֤���ɵ���Һ�к�Cu2+����ȴ��A����Һ����ʢˮ���ձ��й۲��Ƿ������ɫ����������ɫ��Һ֤������Cu2+��
��5����������ԭ��Ӧ�е����غ���ƽ��ѧ����ʽ�ɵ�a:b=5:4��
��6��n(Na2S2O3)=0.025L��0.36mol/L=0.009mol
����������ͭ�����ʵ���Ϊn,�ɷ�Ӧ![]()
��Cu��+----2 S2O3��-
2 2
n 0.009mol��250/25
���n��0.09mol��6.4gͭ��ȫת��Ϊ����ͭ�����ʵ���Ϊ6.4g/64g��mol-1=0.1mol����������ͭ����Ϊ90%��

 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�