��Ŀ����
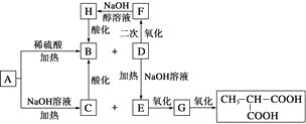
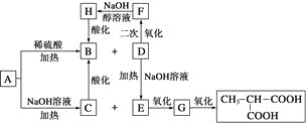
����Ŀ���������أ�KH2PO4����һ�ָ�Ч���Ϸʡ���ҵ��������[��Ҫ�ɷ���Ca3(PO4)2������������Fe2O3��CaF2������]Ϊԭ�ϣ������������ص�������ͼ��

��֪����TBP��D2EHPA��TOA����������ˮ��Һ̬�л������ȡ�ض������н�ǿ��ѡ���ԣ���������ȡ����
����ȡ��TBP��H3PO4��Fe3+�н�ǿ����ȡ���ã�����Ca2+��һ������ȡ���á�
����ȡ��D2EHPA����Fe3+�н�ǿ����ȡ���á�
�ش��������⣺
��1�������ᡱ��������������Ļ�ѧ����ʽΪ______��
��2����������ʱ������Ӧ�Ļ�ѧ����ʽΪ______��
��3����Ŀ��1����______��
��4����Ŀ��2����������л����г�H3PO4�⣬��������ij���������ӡ�ȥ���л����и������ӵķ������ú�H2SO4������ϴ�ӣ���Ӧ�����ӷ���ʽΪ______��
��5������Ӧ��ʱ���������м���KCl���ټ���TOA��TOA��������______��
��6������Ӧ���У�TOA��������Ӱ����Һ��pH��ˮ��Һ��H3PO4��H2PO4-��HPO42-��PO43-�ķֲ�������������Ԫ����ռȫ���������ӵ����ʵ�����������pH�ı仯��ͼ��ʾ��

����Ӧ���У���pH=______����ѡ��2.2������4.5������9.5����12.4����ʱ��ֹͣ����TOA��
���𰸡�Ca3(PO4)2+6HCl��3CaCl2+2H3PO4 SiO2+4HF��SiF4��+2H2O ��ȥ�����к��е�Fe3+ ![]() ˮ���д���KCl+H3PO4
ˮ���д���KCl+H3PO4![]() HCl+KH2PO4������TOA��HClת�Ƶ��л��㣬ƽ��������Ӧ�����ƶ���������KH2PO4���� 4.5
HCl+KH2PO4������TOA��HClת�Ƶ��л��㣬ƽ��������Ӧ�����ƶ���������KH2PO4���� 4.5
��������
������[��Ҫ�ɷ���Ca3(PO4)2������������Fe2O3��CaF2������]Ϊԭ�ϣ������������أ������̿�֪�������ᷢ��Ca3(PO4)2+6HCl��3CaCl2+2H3PO4��Fe2O3+6HCl��2FeCl3+3H2O��CaF2+2HCl��CaCl2+2HF��������Զ������跢��SiO2+4HF=SiF4��+2H2O��D2EHPA����Fe3+�н�ǿ����ȡ���ÿɳ�ȥ�����к��е�Fe3+����Һȡˮ���TBP��ȡH3PO4����Һȡ�л��������������ᣬ����KCl��ϡ��������KH2PO4���ټ����л���-������(TOA)���룬��ˮ��ᾧ�ɵ�KH2PO4��Ʒ���Դ������
��1�������ᡱ��������������Ļ�ѧ����ʽΪCa3(PO4)2+6HCl��3CaCl2+2H3PO4��
��2��HF����������跴Ӧ��������ʱ������Ӧ�Ļ�ѧ����ʽΪSiO2+4HF��SiF4��+2H2O��
��3��������ȡ��D2EHPA����Fe3+�н�ǿ����ȡ���ã���ˡ�Ŀ��1���dz�ȥ�����к��е�Fe3+��
��4����Ŀ��2����������л����г�H3PO4�⣬��������ij���������Ӹ����ӡ�ȥ���л����и������ӵķ������ú�H2SO4������ϴ�ӣ���Ӧ�����ӷ���ʽΪ![]() ��
��
��5������Ӧ��ʱ���������м���KCl���ټ���TOA������ˮ���д���KCl+H3PO4![]() HCl+KH2PO4������TOA��HClת�Ƶ��л��㣬ƽ��������Ӧ�����ƶ���������KH2PO4���ɣ�
HCl+KH2PO4������TOA��HClת�Ƶ��л��㣬ƽ��������Ӧ�����ƶ���������KH2PO4���ɣ�
��6����ͼ��֪��BΪ����![]() ����������pH������
����������pH������![]() ��������pH=4.5ʱ��
��������pH=4.5ʱ��![]() ��࣬pH�����ߣ�������
��࣬pH�����ߣ�������![]() �����ɣ���pH=4.5��ֹͣ����TOA��
�����ɣ���pH=4.5��ֹͣ����TOA��

����Ŀ��ij������ȤС���H2O2�ķֽ�������������ʵ��̽����
(1)�±��Ǹ�С���о�Ӱ���������(H2O2)�ֽ����ʵ�����ʱ�ɼ���һ�����ݣ���10mL H2O2��ȡ150mLO2�����ʱ��(��)
| 30% H2O2 | 15% H2O2 | 10% H2O2 | 5% H2O2 |
������������ | ��������Ӧ | ��������Ӧ | ��������Ӧ | ��������Ӧ |
���������� | 360 | 480 | 540 | 720 |
MnO2���������� | 10 | 25 | 60 | 120 |
�ٸ��о�С������Ʒ���ʱ��������Ũ�ȡ�___________��____________�����ضԹ�������ֽ����ʵ�Ӱ�졣
�ڴ�����Ӱ���������ֽ����ʵ���������ѡһ����˵�������ضԷֽ������к�Ӱ�죿_______________________��
(2)��������ͬ���ۼ�״̬��ͬ��MnO2�ֱ���뵽5mL 5%(�ܶ�Ϊ1.0g/cm3)��˫��ˮ�У����ô����ǵ�ľ�����ԡ��ⶨ������£�
ʵ�� ��� | ������MnO2�� | ���� ��� | �۲��� | ��Ӧ��� �����ʱ�� |
A | ��ĩ״ | ��ϲ��� | ���ҷ�Ӧ�������ǵ�ľ����ȼ | 5span>���� |
B | ��״ | ��Ӧ���������Ǻ�����ľ��δ��ȼ | 30���� |
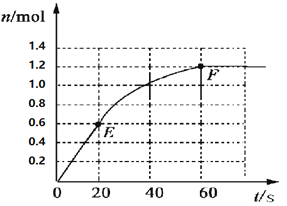
�� д��H2O2�����ֽ�Ļ�ѧ��Ӧ����ʽ________________�����ʵ��A��H2O2��5�����ڵ�ƽ����Ӧ����________________�����������С�������λ���֣�
�� ʵ����˵���������õĴ�С��____________________�йء�