��Ŀ����
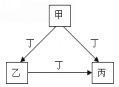
����Ŀ��A��B��C��D��Ϊ��ѧ��ѧ�ij��������Ҿ�����ͬһ��Ԫ�أ�����֮���ת����ϵ����ͼ��ʾ(��Ӧ���������������Ѿ���ȥ)��
![]()
��1����A��һ�ֽ�����C�ǵ���ɫ���壬д��C��һ����;_______��
��2����AΪ����ɫ���嵥�ʣ�д��D��Ũ��Һ��ͭ��Ӧ�Ļ�ѧ����ʽ______��
��3����A�ǻ����C�Ǻ���ɫ���壬��A�Ļ�ѧʽΪ_____________��Cת��ΪD�Ĺ����У��������뻹ԭ����������Ϊ__________________��
���𰸡� �������DZˮͧ�й����� 2H2SO4(Ũ)��Cu![]() CuSO4��SO2����2H2O NH3 1:2
CuSO4��SO2����2H2O NH3 1:2
����������1����A��һ�ֽ�����C�ǵ���ɫ���壬��A��Na��B�������ƣ�C�ǹ������ƣ�D���������ƣ��������Ƶ�һ����;�����Ǻ������DZˮͧ�й���������2����AΪ����ɫ���嵥�ʣ���A��S��B��SO2��C��SO3��D�����ᣬD��Ũ��Һ��ͭ��Ӧ�Ļ�ѧ����ʽΪ2H2SO4(Ũ)��Cu![]() CuSO4��SO2����2H2O����3����A�ǻ����C�Ǻ���ɫ���壬��A��NH3��B��NO��C��NO2��D�����ᣬCת��ΪD�ķ���ʽΪ3NO2+H2O��2HNO3+NO������NO2������������Ҳ�ǻ�ԭ�����������������NO�ǻ�ԭ����������뻹ԭ����������Ϊ1:2��
CuSO4��SO2����2H2O����3����A�ǻ����C�Ǻ���ɫ���壬��A��NH3��B��NO��C��NO2��D�����ᣬCת��ΪD�ķ���ʽΪ3NO2+H2O��2HNO3+NO������NO2������������Ҳ�ǻ�ԭ�����������������NO�ǻ�ԭ����������뻹ԭ����������Ϊ1:2��

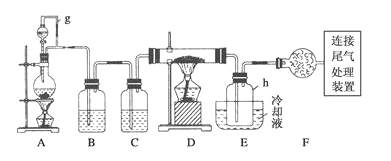
����Ŀ��ͨ����̼�ڸ����»�ԭ���������Ƶôֹ裬�ֹ�(������������������)��������Ӧ�������Ȼ���(��Ӧ�¶�450-500��)���������辭�ᴿ����������ԭ�ɵøߴ��衣������ʵ�����Ʊ����Ȼ����װ��ʾ��ͼ��(D��Ӳ�ʲ�������ʢװ�ֹ�)
�����Ϣ����:a.����������ˮ����Ӧ���ɹ�����Ȼ��⣻
b.�������������ڸ����¾���������ֱ�ӷ�Ӧ������Ӧ���Ȼ��
c.�й����ʵ������������±�:
���� | SiCl4 | BCl3 | AlCl3 | FeCl3 | PCl5 |
�е�/�� | 57.7 | 12.8 | �� | 315 | �� |
�۵�/�� | ��70.0 | ��107.2 | �� | �� | �� |
�����¶�/�� | �� | �� | 180 | 300 | 162 |
��ش���������:
(1)д����̼�ڸ����»�ԭ���������Ƶôֹ�Ļ�ѧ����ʽ_________________________��
(2)д��װ��A�з�����Ӧ�����ӷ���ʽ__________________________________��
(3)װ��A��g�ܵ�������_________________��װ��C�е��Լ���____________��
(4)װ��E��hƿ�ռ����Ĵֲ����ͨ���������õ��ߴ������Ȼ��裬�����IJ������У�����Ԫ������ܻ����е�����Ԫ����________(��дԪ�ط���)��
(5)д��β������װ���з�����Ӧ�����ӷ���ʽ___________________________��
(6)����ʵ��װ������һ���ԵIJ���֮�����������ĸĽ�����������: ___________��
����Ŀ���ڼס��ҡ���������ͬ�ܱ������а���ͬ��ʽͶ�ϣ�һ�������·�����Ӧ(��ʼ�¶Ⱥ���ʼ�����ͬ):N2(g)+3H2(g)![]() 2NH3(g) ��H<0������������±���ʾ:
2NH3(g) ��H<0������������±���ʾ:
���� | �� | �� | �� |
������� | ���º��� | ���Ⱥ��� | ���º�ѹ |
��Ӧ��Ͷ�� | lmolN2��3molH2 | 2molNH3 | 2molNH3 |
ƽ��ʱ������� | V�� | V�� | V�� |
��Ӧ��ƽ�ⳣ��K | K�� | K�� | K�� |
ƽ��ʱNH3��Ũ��/mol/L | c�� | c�� | c�� |
ƽ��ʱNH3�ķ�Ӧ����/mol/(L��min) | v�� | v�� | v�� |
����˵����ȷ����
A. V��>V�� B. K��>K�� C. c��>c�� D. V��=V��