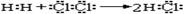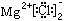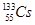��Ŀ����
��8�֣�ijͬѧ�����в�������500 mL 0.2 mol��L��1 KCl��Һ����ش��й����⡣
| ʵ�鲽�� | �й����� |
| �ټ�������KCl������ | ��ҪKCl������Ϊ________g(����С�����һλ) |
| �ڳ���KCl���� | ������Ҫ�õ�����Ҫ������________________ |
| �۽�KCl����100 mL�ձ��У�����������ˮ | Ϊ�˼ӿ��ܽ����ʣ����Բ�ȡ��Щ��ʩ�� ________________ |
| �ܽ��ձ�����Һת����500 mL����ƿ�� | Ϊ�˷�ֹ��Һ������Ӧ��ȡʲô��ʩ�� __________________ |
| ��������ƿ�м�����ˮ���̶��� | �ڽ��д˲���ʱ����ˮ����̶���1 cm��2 cm��Ӧ��β�����____________________ |
��2���ڽ��Тܲ�����ʱδ��ϴ���ձ�����������ϴ��Һת��������ƿ����������ҺŨ��
________(�ƫ�ߡ�����ƫ�͡���Ӱ�족)��
��3�������Тݲ�����ʱ������ˮ�����̶��ߣ�����________��
��7.5����������ƽ��ҩ�ס��۽���(���ʵ�����)�����ò���������������
�ݸ��ý�ͷ�ιܼ�ˮ����Һ����̶�������
��1������ƿ�Ƿ�©ˮ����2��ƫ�ͣ�3����������
������������� ��������֪��500mL 0.2mol?L-1KCl��Һ�к��������Ȼ���0.1mol����Ҫ�Ȼ��ص�����Ϊ��74.5g/mol��0.1mol=7.45��7.5g������7.5g�Ȼ��أ�����ʹ��������ƽ���г�����Ϊ�˼����ܽ⣬��Ҫʹ�ò��������н��裻ת����Һʱ����Ҫʹ�ò�����������Ŀ���DZ���Һ����������ƿ��ߣ�����ʱ����ֱ�Ӽ�����ˮ������ƿ�̶���1-2cm�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������У���1����ʹ������ƿ����һ�����ʵ���Ũ�ȵ���Һʱ������������ƿ�Ƿ�©ˮ����2��δ��ϴ���ձ���������ϴҺת��������ƿ���������Ƶ���Һ�����ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ���3�������Тݲ�����ʱ������ˮ�����̶��ߣ��ᵼ�����Ƶ���ҺŨ��ƫ�ͣ��˴�����ʧ�ܣ���Ҫ�����������ơ�
���㣺����������һ�����ʵ���Ũ�ȵ���Һ��������������

 ��У����ϵ�д�
��У����ϵ�д���NAΪ�����ӵ�������ֵ������˵����ȷ����
| A��1L0.5mol��L��1 ��(NH4)2SO4��Һ�к�NH4+��ΪNA |
| B�����³�ѹ�£�1molCH4�к���4NA��C��H�� |
| C�����³�ѹ�£�48g O3����ԭ����Ϊ2NA�����ԭ��������O 16�� |
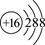
| D����״���£�22.4LC6H6(��)�ķ�����ĿΪNA |
��һ��������Ϊ�ص�KOH��Һ������m gˮ����������ǡ�ñ�Ϊ3�أ����ΪVL(��Һ����������)����Ũ������Һ�����ʵ���Ũ��Ϊ�� ��
A�� mol��L-1 mol��L-1 | B�� mol��L-1 mol��L-1 |
C�� mol��L-1 mol��L-1 | D�� mol��L-1 mol��L-1 |