��Ŀ����
����Ŀ����ϩ�Ƿdz���Ҫ���л�����IJ���ͨ����������һ������ʯ�ͻ�����չˮƽ����ش��������⣺
��1����ҵ������ϩΪԭ�Ͽɺϳ�һ��Ҫ�л��߷��ӻ�����ò��Ͽ�����ʳƷ��װ���ϳɸ����ʵĻ�ѧ����ʽΪ________����Ӧ������_____��
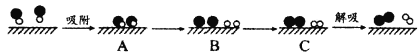
��2��Ϊ̽��ʵ��������ϩ����ϩ����ˮ�ķ�Ӧ����ͬѧ�������ͼ��ʾ��ʵ��װ�á��Ʊ���ϩ�Ļ�ѧ����ʽ��________����ϩʹ��ˮ��ɫ�Ļ�ѧ����ʽ��__________����Ӧ����_____��
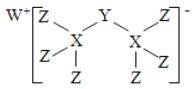
��3��д��������������Ӧ��ͬ���칹��Ľṹ��ʽ��
��![]() ��ͬ���칹��Ľṹ��ʽ____________________��
��ͬ���칹��Ľṹ��ʽ____________________��
��![]() ��ͬ���칹��Ľṹ��ʽ____________________��
��ͬ���칹��Ľṹ��ʽ____________________��
���𰸡�nCH2=CH2![]()
![]() �Ӿ۷�Ӧ C2H5OH
�Ӿ۷�Ӧ C2H5OH![]() CH2=CH2��+H2O CH2=CH2+Br2��BrCH2CH2Br �ӳɷ�Ӧ (CH3)2CHCH3 CH2=CHCH3
CH2=CH2��+H2O CH2=CH2+Br2��BrCH2CH2Br �ӳɷ�Ӧ (CH3)2CHCH3 CH2=CHCH3
��������
(1) ʳƷ��װ�IJ����Ǿ���ϩ��������Ӧ�Ļ�ѧ����ʽΪ
nCH2=CH2![]()
![]() ����nCH2=CH2
����nCH2=CH2![]()
![]()
��Ӧ����Ϊ�Ӿ۷�Ӧ����Ϊ���Ӿ۷�Ӧ
(2) �Ʊ���ϩ�Ļ�ѧ����ʽ��C2H5OH![]() CH2=CH2��+H2O
CH2=CH2��+H2O
����C2H5OH![]() CH2=CH2��+H2O
CH2=CH2��+H2O
��ϩʹ��ˮ��ɫ�Ļ�ѧ����ʽ��CH2=CH2+Br2��BrCH2CH2Br
����CH2=CH2+Br2��BrCH2CH2Br
��Ӧ���ͼӳɷ�Ӧ����Ϊ���ӳɷ�Ӧ
(3) ��![]() ��ͬ���칹��Ľṹ��ʽΪ(CH3)2CHCH3��
��ͬ���칹��Ľṹ��ʽΪ(CH3)2CHCH3��
��ΪΪ��(CH3)2CHCH3
��![]() ��ͬ���칹��Ľṹ��ʽΪCH2=CHCH3����Ϊ��CH2=CHCH3
��ͬ���칹��Ľṹ��ʽΪCH2=CHCH3����Ϊ��CH2=CHCH3

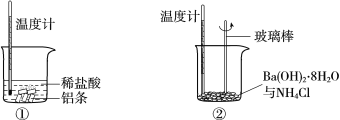
����Ŀ��ij��ѧѧϰС���������ʵ�鷽�����ⶨij����NaCl��С�մ���Ʒ��![]() ������������
������������
������һ�������ϣ�NaCl������![]() ʱ�ۻ������ֽ⣬
ʱ�ۻ������ֽ⣬![]() ���ȷֽ⣬
���ȷֽ⣬![]() ���ɴ����ʵ�飺�õ�����ƽ��ȡ
���ɴ����ʵ�飺�õ�����ƽ��ȡ![]() ��Ʒ�������������þƾ��Ƽ��ȣ���ͼ�������¶ȸ���
��Ʒ�������������þƾ��Ƽ��ȣ���ͼ�������¶ȸ���![]() ��������
��������![]() ���������غ���ȴ������ʣ���������Ϊ
���������غ���ȴ������ʣ���������Ϊ![]() ��
��
��1��ʵ�������ٳ���____�Ρ�
��2��ʵ���У��ﵽ���ز����ı���__________��
����������������![]() ��Һ�ʼ��ԣ�
��Һ�ʼ��ԣ�![]() �����������ʵ�飺ȷ��ȡ1.000 g��Ʒ��������ƿ���100 mL��Һ���õζ�����ȡ20.00 mL����ƿ�У�����2�μ���Ϊָʾ������0.1000mol/L�������Һ�ζ���ƽ�����ݣ�����ʵ����������£�
�����������ʵ�飺ȷ��ȡ1.000 g��Ʒ��������ƿ���100 mL��Һ���õζ�����ȡ20.00 mL����ƿ�У�����2�μ���Ϊָʾ������0.1000mol/L�������Һ�ζ���ƽ�����ݣ�����ʵ����������£�
1 | 2 | |
| 20.00 | 20.00 |
| 0.00 | 0.20 |
| 19.98 | 20.22 |
��3��ʵ���У���������������ȷʱ�����в�����������ʵ����������______����
A������ƿ������ˮϴ����ƿ����ˮ������ֱ��������Һ
B���ζ����ڱ���ˮ���װ���Һ
C����ƿ�ڱ���ˮ�飬�ô���Һ��ϴ����ʹ��
D����ƿ������ˮϴ����ֱ�ӷ������Һ���вⶨ
span>��4���ζ��յ���жϣ�__________��
��5������ʵ���������������ƽ��ֵΪ__________mL��
��6����Ʒ��![]() ����������__________��
����������__________��