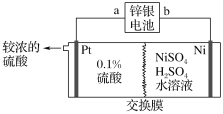
��Ŀ����
����Ŀ��![]() ���������ƣ�������ˮ��ˮ��Һ�����ԣ�����ǿ��ԭ�ԣ������ڻ�ѧ��������������.һ���������ף���
���������ƣ�������ˮ��ˮ��Һ�����ԣ�����ǿ��ԭ�ԣ������ڻ�ѧ��������������.һ���������ף���![]() ������
������![]() ��
��![]() ��
��![]() ��
��![]() �ȣ�Ϊԭ���Ʊ�
�ȣ�Ϊԭ���Ʊ�![]() �Ĺ����������£�
�Ĺ����������£�
��֪����![]() �����ּ�ķ�Ӧ��Ҫ�У�
�����ּ�ķ�Ӧ��Ҫ�У�
I.![]()
II.![]()
III.![]()
��2.ʵ���¶��£�![]()
![]()
��1����֪![]() ��һԪ��ǿ�ᣬд��
��һԪ��ǿ�ᣬд��![]() ��Һ�д��ڵ�����ƽ�ⷽ��ʽ��_________��
��Һ�д��ڵ�����ƽ�ⷽ��ʽ��_________��
��2��ͨ![]() ����
����![]() ��ʱ��д����ȥ��Һ��
��ʱ��д����ȥ��Һ��![]() ���ʵ����ӷ���ʽ��_________��
���ʵ����ӷ���ʽ��_________��
��3��������2��������2��Ҫ�ɷ�Ϊ_________���ѧʽ��������������ȥ![]() ��
��![]() ��ʱ�����õ�
��ʱ�����õ�![]() ��
��![]() ��
��![]() ��Һ��������һ������
��Һ��������һ������![]() ��Һ���ã���Һ��
��Һ���ã���Һ��![]() �����ʱ��Һ��
�����ʱ��Һ��![]() ��Ũ��Ϊ_________
��Ũ��Ϊ_________![]() ��
��
��4��β���е�![]() ��
��![]() ��Һ��������
��Һ��������![]() ��������շ�Ӧ�����������뻹ԭ��������ʵ���֮��Ϊ_________��������Һ�о��ᾧ�����ˡ�ϴ�ӡ����Ҳ�ɻ�ò�Ʒ
��������շ�Ӧ�����������뻹ԭ��������ʵ���֮��Ϊ_________��������Һ�о��ᾧ�����ˡ�ϴ�ӡ����Ҳ�ɻ�ò�Ʒ![]() ��ϴ�Ӹò�Ʒ�����Ҵ�����ԭ����_________��
��ϴ�Ӹò�Ʒ�����Ҵ�����ԭ����_________��
��5���������Ƶ���Ԫ���ױ�ǿ�����������������ʵ���ҿ��õζ����ⶨ��Ʒ������
��ͬѧȷ��ȡ![]() ��Ʒ���
��Ʒ���![]() ��Һȷ��ȡ
��Һȷ��ȡ![]() ��Һ����ƿ�У���
��Һ����ƿ�У���![]() ����
����![]() ����Һ���淶�����ܵĶ��ƽ�еζ���ƽ����������
����Һ���淶�����ܵĶ��ƽ�еζ���ƽ����������![]() ��Һ
��Һ![]() �����ò�Ʒ�Ĵ���Ϊ_________����ͬѧ��Ϊ��ͬѧ�IJⶨ�������ѧ���������¼�ͬѧ�ó���˲ⶨ����������ԭ����_________��
�����ò�Ʒ�Ĵ���Ϊ_________����ͬѧ��Ϊ��ͬѧ�IJⶨ�������ѧ���������¼�ͬѧ�ó���˲ⶨ����������ԭ����_________��
���𰸡�![]() ��
��![]()
![]()
![]() ��
��![]()
![]() 1:2 ϴȥ���������ʣ����ٲ�Ʒ
1:2 ϴȥ���������ʣ����ٲ�Ʒ![]() ���ܽ���ʧ���Ҵ��ӷ������ڸ���. 106%
���ܽ���ʧ���Ҵ��ӷ������ڸ���. 106% ![]() ������ȥ�ᾧˮ
������ȥ�ᾧˮ
��������
���ף���![]() ������
������![]() ��
��![]() ��
��![]() ��
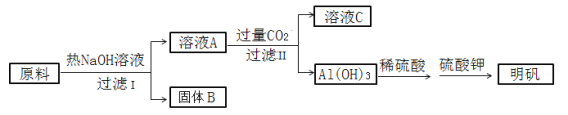
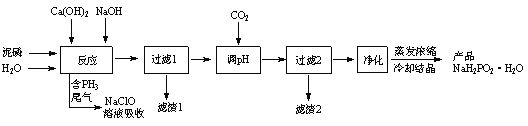
��![]() �ȣ��м���NaOH��Һ��Ca(OH)2��Һ�����˷���P4��NaOH��Ca(OH)2�ķ�Ӧ�⣬���ᷢ��Al2O3���ܽⷴӦ��CaO��ˮ��ΪCa(OH)2��CaCl2��������Һ�У�Fe2O3����뵽����1�У�����Һ1��ͨ��CO2��Ca2+ת��ΪCaCO3������AlO2-ת��ΪAl(OH)3����Һ2������������ȥCl-��
�ȣ��м���NaOH��Һ��Ca(OH)2��Һ�����˷���P4��NaOH��Ca(OH)2�ķ�Ӧ�⣬���ᷢ��Al2O3���ܽⷴӦ��CaO��ˮ��ΪCa(OH)2��CaCl2��������Һ�У�Fe2O3����뵽����1�У�����Һ1��ͨ��CO2��Ca2+ת��ΪCaCO3������AlO2-ת��ΪAl(OH)3����Һ2������������ȥCl-��![]() �ȣ��õ���Ʒ��
�ȣ��õ���Ʒ��
��1�����и�֪H3PO2��һԪ��ǿ�ᣬ��NaH2PO2��Һ�д���H2PO2-��ˮ�⣬�Լ�ˮ�ĵ��룻
��2��ͨ��CO2������ҺpHʱ����Һ�к���Ca2+��OH-���ݴ˿���д����ȥCa2+�����ӷ���ʽ��
��3������2�г�����CaCO3�⣬������Al(OH)3���������ӻ��ɼ������Һ��CO32-��Ũ�ȣ�
��4�����ݵ�ʧ�����غ���Լ�����������ͻ�ԭ��������ʵ���֮�ȣ����Ҵ�ϴ�Ӳ�Ʒ������Ϊ�Ҵ����Լ��ٲ�Ʒ�ܽ����ʧ�����Ҵ��ӷ��������ڸ��
��5��������������Ƴ��÷�Ӧ�����ӷ���ʽΪ2H++4MnO4-+5H2PO2-=5PO43-+4Mn2++6H2O������չ����ؼ��㡣
��1��H3PO2��һԪ��ǿ�ᣬ��NaH2PO2��Һ�д���H2PO2-��ˮ�⣬�Լ�ˮ�ĵ��룬�ʸ���Һ�е�ƽ�ⷽ��ʽ�У�![]() ��
��![]() ��
��
��2��ͨ��CO2������ҺpHʱ����Һ�к���Ca2+��OH-�����ȥCa2+�����ӷ���ʽΪ��![]() ��
��
![]() =
=![]() =1.0��10-3mol��L-1��c(CO32-)=
=1.0��10-3mol��L-1��c(CO32-)=![]() =
=![]() =2.5��10-6mol��L-1��
=2.5��10-6mol��L-1��
��4��PH3��NaClO��Һ���գ�PH3����ԭ��������NaH2PO2ʱ��ת��4�����ӣ�NaClO��������������NaCl��ת��2�����ӣ����ڷ�Ӧ�е�ʧ���Ӹ�����ͬ������������NaH2PO4�ͻ�ԭ����NaCl�����ʵ���֮��Ϊ1��2�������Ҵ�ϴ�Ӳ�Ʒ������Ϊϴȥ���������ʣ����ٲ�Ʒ![]() ���ܽ���ʧ���Ҵ��ӷ������ڸ��
���ܽ���ʧ���Ҵ��ӷ������ڸ��
��5��20mL��Һ���ζ�ʱ��n(KMnO4)=0.2mol��L-1��0.02L=0.004mol����Ϊ4MnO4-~5H2PO2-�� n(H2PO2-)=0.05mol��������100mL��Һ����NaH2PO2��H2O 0.25mol���ʸò�Ʒ�Ĵ���Ϊ��![]() =106%����Ʒ�Ĵ��Ȳ����ܴ���100%�������еĴ���ƫ�������������£�ԭ��������Ϊ��Ʒ��������ƫС������Ʒ����ʧˮ��
=106%����Ʒ�Ĵ��Ȳ����ܴ���100%�������еĴ���ƫ�������������£�ԭ��������Ϊ��Ʒ��������ƫС������Ʒ����ʧˮ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�