��Ŀ����
6��ͬ���칹�������л���ѧ���ձ���ڣ���ѧ�γ�����ͬ���칹�������ࣺ�ٹ���������칹���ڹ�����λ���칹����̼�Ǽ��칹����֪����ʽΪC5H12O���л����ж���ͬ���칹�壬��������������֣�
����������Ϣ����������⣺
��1�����������������ж�A���ڴ����л������B��C��D�У���A��Ϊ�������칹����B ������ţ���ͬ������A��Ϊ̼���칹����D����A��Ϊ������λ���칹����C��
��2��д����һ����A��Ϊ������λ���칹���л������дC���Ľṹ��ʽ��
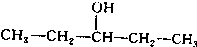 ��
����3����A��Ϊ̼���칹��ͬ���칹�干��5�֣���B��C��D�е�һ�����⣬�������ֽṹ��ʽΪ��
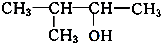 ��
�� д����������ͬ���칹��Ľṹ��ʽ��
д����������ͬ���칹��Ľṹ��ʽ��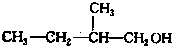 ��
��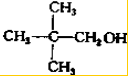 ��
��
���� ��1���������ʹ��������жϣ��������칹��ָ����Ĺ����Ų�һ����ͬ���칹�壻̼���칹��ָ���еĹ�����һ��������̼����һ����ͬ���칹����л��������λ���칹��ָ�����ŵ�λ�ò�ͬ�����ǹ�������ͬ���칹�壻
��2����Ϊ������λ���칹���л���ֻҪ�������ŵ�λ�ñ仯���ɵõ���
��3������̼���칹����д�������ش�ע�������̼���칹����д������
��� �⣺��1��A�к��д��ǻ������ڴ��࣬B��A��Ϊ�������칹���ǣ�D��A��Ϊ̼���칹��C��A��Ϊ������λ���칹���ʴ�Ϊ������B��D��C��
��2��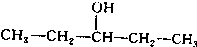 ��A��Ϊ������λ���칹���ʴ�Ϊ��
��A��Ϊ������λ���칹���ʴ�Ϊ��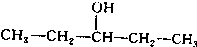 ��
��
��3����A��Ϊ̼���칹��ͬ���칹�壬���Ը��������̼���칹����д�������CH3CH2CH2CH2CH2-��CH3CH2CH2CH��CH3��-��CH3CH2CH��CH2CH3��-����CH3��2CHCH2CH2-����CH3��3CCH2-��������������ͬ���칹��Ľṹ��ʽΪ��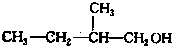 ��
�� ���ʴ�Ϊ��
���ʴ�Ϊ��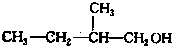 ��
�� ��
��
���� ���⿼��ѧ��ͬ���칹�������Լ��칹�����д��֪ʶ��ע��֪ʶ�Ĺ��ɺ������ǹؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
16����50mL1.0mol/L������50mL1.1mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺

��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ�ǻ��β��������������Ʒ�ܷ���������Ʒ���治�ܣ�ԭ���Ǣ����ĵ���ϵ��������ɢ�ȣ������������ᷴӦ
��2��ͼʾ����Ʒ��Ӻ��װ�ý���������
��3���ձ���������ֽ���������DZ��£�����������ɢʧ��
��4�����ձ����粻��Ӳֽ�壬��õ��к�����ֵƫС���ƫ��ƫС����Ӱ�족����
��5�������60mL1.0mol/L������50mL1.1mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�����������ȣ����ȡ�����ȡ����������к�����ȣ����ȡ�����ȡ�������֪��ϡ��Һ��ǿ����ǿ����кͷ�Ӧ����1molˮʱ�ų�57.3kJ��������д��������Ӧ���Ȼ�ѧ����ʽNaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l����H=-57.3 kJ/mol
��6������ͬŨ�Ⱥ�����İ�ˮ��NH3•H2O������NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��ƫС�����ƫ����ƫС��������Ӱ�족����
��7����ʵ�������Թ�����NaOH��ԭ��̲���˵��Ϊ��֤������ȫ���кͣ����ʣ������ڷ�Ӧ������Ϊ�з�������������������ڷ�Ӧ�лӷ������õ��к�����ֵƫ�ͣ���ƫ�ߡ�ƫ�ͻ䣩��
��8����ʵ��С����������ʵ�飬ÿ��ȡ��Һ��50mL������¼���ԭʼ���ݣ�
��֪���ᡢNaOH��Һ�ܶȽ���Ϊ1.00g/cm3�кͺ���Һ�ı����� C=4.18J��g��•�棩��Q=C•m•��t��÷�Ӧ���к���Ϊ��H=-56.0kJ/mol��

��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ�ǻ��β��������������Ʒ�ܷ���������Ʒ���治�ܣ�ԭ���Ǣ����ĵ���ϵ��������ɢ�ȣ������������ᷴӦ
��2��ͼʾ����Ʒ��Ӻ��װ�ý���������
��3���ձ���������ֽ���������DZ��£�����������ɢʧ��
��4�����ձ����粻��Ӳֽ�壬��õ��к�����ֵƫС���ƫ��ƫС����Ӱ�족����
��5�������60mL1.0mol/L������50mL1.1mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�����������ȣ����ȡ�����ȡ����������к�����ȣ����ȡ�����ȡ�������֪��ϡ��Һ��ǿ����ǿ����кͷ�Ӧ����1molˮʱ�ų�57.3kJ��������д��������Ӧ���Ȼ�ѧ����ʽNaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l����H=-57.3 kJ/mol
��6������ͬŨ�Ⱥ�����İ�ˮ��NH3•H2O������NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��ƫС�����ƫ����ƫС��������Ӱ�족����
��7����ʵ�������Թ�����NaOH��ԭ��̲���˵��Ϊ��֤������ȫ���кͣ����ʣ������ڷ�Ӧ������Ϊ�з�������������������ڷ�Ӧ�лӷ������õ��к�����ֵƫ�ͣ���ƫ�ߡ�ƫ�ͻ䣩��
��8����ʵ��С����������ʵ�飬ÿ��ȡ��Һ��50mL������¼���ԭʼ���ݣ�
| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶ȣ�t2���� | �²t2-t1���� | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
17����֪XΪFeO��CuO�Ļ���ȡ���ݵ�������X��Ʒ��������ʵ�飺���3�����ù���D������Ϊ 32g����ҺE��ֻ����һ�ֽ������ӣ�����F�ڱ�״�������Ϊ5.6 L����X��FeO��CuO�����ʵ� ��֮��Ϊ��������
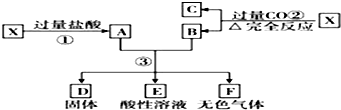

| A�� | 4��1 | B�� | 1��2 | C�� | 2��1 | D�� | 1��1 |
11��X��Y��Z����ǿ����ʣ�������ˮ�е�������������±���ʾ��
��ͼ1��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��250mL������X��Һ��������Y��Һ��������Z��Һ���缫��Ϊʯī�缫����ͨ��Դ������һ��ʱ��������c�缫��������6.4g�������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵͼ2���£��ݴ˻ش��������⣺
��1��MΪ��Դ�ĸ�������д�����������������һ��ʱ�����e��f���������ֱ�μӷ�̪��������e����
��2�����ձ��е缫b�Ϸ����ĵ缫��ӦΪ4OH--4e-=O2��+2H2O��
��3�����ձ������ܷ�Ӧ���ӷ���ʽΪ2Cu2++2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2Cu+O2��+4H+�����ձ���a��b������������״����3.36L�����壮
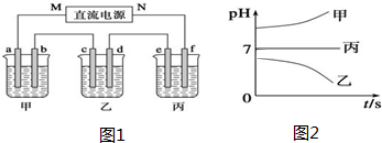
��ͼ1��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��250mL������X��Һ��������Y��Һ��������Z��Һ���缫��Ϊʯī�缫����ͨ��Դ������һ��ʱ��������c�缫��������6.4g�������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵͼ2���£��ݴ˻ش��������⣺
| ������ | Na+��K+��Cu2+ |
| ������ | SO42-��OH- |
��2�����ձ��е缫b�Ϸ����ĵ缫��ӦΪ4OH--4e-=O2��+2H2O��
��3�����ձ������ܷ�Ӧ���ӷ���ʽΪ2Cu2++2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2Cu+O2��+4H+�����ձ���a��b������������״����3.36L�����壮

18��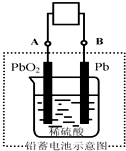 ��ƿ�����õ��һ��ΪǦ���أ�����һ�ֵ��͵Ŀɳ���أ�����ܷ�ӦʽΪ��2PbSO4+2H2O $?_{�ŵ�}^{���}$Pb+PbO2+4H++2SO42-��������˵����ȷ���ǣ�������
��ƿ�����õ��һ��ΪǦ���أ�����һ�ֵ��͵Ŀɳ���أ�����ܷ�ӦʽΪ��2PbSO4+2H2O $?_{�ŵ�}^{���}$Pb+PbO2+4H++2SO42-��������˵����ȷ���ǣ�������
 ��ƿ�����õ��һ��ΪǦ���أ�����һ�ֵ��͵Ŀɳ���أ�����ܷ�ӦʽΪ��2PbSO4+2H2O $?_{�ŵ�}^{���}$Pb+PbO2+4H++2SO42-��������˵����ȷ���ǣ�������
��ƿ�����õ��һ��ΪǦ���أ�����һ�ֵ��͵Ŀɳ���أ�����ܷ�ӦʽΪ��2PbSO4+2H2O $?_{�ŵ�}^{���}$Pb+PbO2+4H++2SO42-��������˵����ȷ���ǣ�������| A�� | �ŵ�ʱ�����ӷ�����B��A | |
| B�� | �ŵ�ʱ��������Ӧ�� Pb-2e-+SO42-�TPbSO4 | |
| C�� | ���ʱ��������Ӧ��PbSO4+2H2O-2e-�TPbO2+SO42-+4H+ | |
| D�� | ���ʱ��Ǧ���صĸ���Ӧ��������Դ���������� |
15���������ӷ���ʽ����ȷ���ǣ�������
| A�� | ̼�����ϡ�����ϣ�CaCO3+2H+�TCa2++CO2��+H2O | |
| B�� | ����ͭ��Һ���ռ���Һ��ϣ�Cu2++2OH-�TCu��OH��2�� | |
| C�� | ̼��������ϡ�����ϣ�HCO3-+H+�TCO2��+H2O | |
| D�� | �Ѷ�����̼ͨ���Ȼ�����Һ�У�Ca2++H2O+CO2�TCaCO3��+2H+ |
16�����������ж�������Ԫ�أ�������������ǣ�������
| A�� | NH3 | B�� | CH4 | C�� | NaHCO3 | D�� | HNO3 |
 ��Ȼ����â����ѧʽΪNa2SO4•10H2O��Ϊ��ɫ���壬������ˮ����С��ͬѧ���룬���ģ�ҵ�����ӽ���Ĥ�����ռ�ķ���������ͼ��ʾװ�õ����������Һ����ȡ������������������������ƣ����۴ӽ�ʡ��Դ���Ǵ����ԭ�ϵ������ʶ��Զ����ӷ�����ɫ��ѧ���
��Ȼ����â����ѧʽΪNa2SO4•10H2O��Ϊ��ɫ���壬������ˮ����С��ͬѧ���룬���ģ�ҵ�����ӽ���Ĥ�����ռ�ķ���������ͼ��ʾװ�õ����������Һ����ȡ������������������������ƣ����۴ӽ�ʡ��Դ���Ǵ����ԭ�ϵ������ʶ��Զ����ӷ�����ɫ��ѧ��� ��
��