��Ŀ����
�������ƣ�NaClO2��������ˮ��������ɰ�ǡ���֬��Ư����ɱ�����������ù������ⷨ�����������ƵĹ�������ͼ��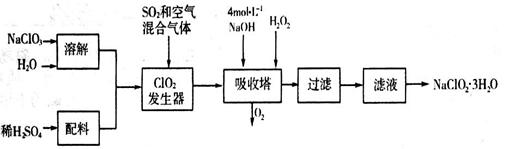
��֪��NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O��
��1���������ڷ�����Ӧ�Ļ�ѧ����ʽΪ_____________________����ƽ��ѧ����ʽ�����ù��������е�NaClO3��ClO2��NaClO2����ǿ�����������Ƕ��ܺ�Ũ���ᷴӦ��ȡCl2�����ö������Ⱥ�Ũ������ȡCl2��������5 mol Cl2ʱ��ͨ����ԭ��Ӧ�Ƶ�����������Ϊ________�ˡ�
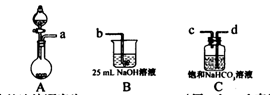
��2������Һ�еõ���NaClO2��3H2O����IJ���������__________����д��ţ���
a.���� b.���� c.���� d.��ȴ�ᾧ
��3��ӡȾ��ҵ�����������ƣ�NaClO2��Ư��֯�Ư��֯��ʱ���������õ���HClO2���±���25��ʱHClO2�����ֳ�������ĵ���ƽ�ⳣ����
�ٳ����£����ʵ���Ũ����ȵ�NaClO2��NaF��NaCN��Na2S������Һ��pH�ɴ�С��˳��Ϊ____________�������ȣ����ʵ���Ũ����ͬ��NaF��NaCN����Һ�������������������Ĵ�С��ϵΪ��______________���ǰ�ߴ���ȡ����ߴ���
��Na2S�dz��õij�������ij��ҵ��ˮ�к��е�Ũ�ȵ�Cu2+��Fe2+��Pb2+���ӣ��μ�Na2S��Һ�����������ij�����_______�������һ�����ӳ�����ȫʱ��������Ũ��Ϊ10-5mol��L-1����ʱ��S2-��Ũ��Ϊ____��
��֪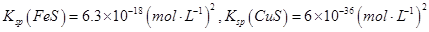
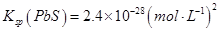
��14�֣���1��2NaOH+2ClO2+H2O2��2NaClO2+2H2O+O2�� 71��ÿ��2�֣���4�֣���2��dc��2�֣�
��3����Na2S��NaCN��NaF��NaClO2 ǰ�ߴ�ÿ��2�֣���4�֣�
��CuS 6.3��10��13mol/L��ÿ��2�֣���4�֣�
���������������1������������Ϣ��֪��������������NaClO2������һ����ClO2��NaClO2�����ϼ۽��ͣ�����ԭ����H2O2�ض�����������������������˷�Ӧ�Ļ�ѧ����ʽΪ2NaOH+2ClO2+H2O2��2NaClO2+2H2O+O2�����������Ⱥ�Ũ������ȡCl2ʱ��������������Ԫ�صĻ��ϼ۴ӣ�4�۽��͵�0�ۣ��õ�4�����ӡ��Ȼ�������Ԫ�صĻ��ϼ۴ӣ�1�����ߵ�0�ۣ�ʧȥ1�����ӣ�����ݵ��ӵĵ�ʧ�غ��֪���������ͻ�ԭ��������ʵ���֮����1:4����ԭ�����������������ʵ���֮��Ҳ��1:4����˵�����5 mol Cl2ʱ��ͨ����ԭ��Ӧ�Ƶ����������ʵ�����1mol������Ϊ71g��
��2������Һ�еõ����ᾧˮ�ľ��壬ֻ�ܲ�ȡ������Ũ������ȴ�ᾧ������ͨ�����˵õ��־��壬���Բ���˳��Ϊdc��
��3������Խ������Ӧ������Խ����ˮ�⣬��Һ�ļ���Խǿ��pHԽ����ĵ���ƽ�ⳣ��ԽС����Խ�������Ը��ݵ���ƽ�ⳣ����֪������ǿ��˳����HClO2��HF��H2S��HCN��HS���������£����ʵ���Ũ����ȵ�NaClO2��NaF��NaCN��Na2S������Һ��pH�ɴ�С��˳��ΪNa2S��NaCN��NaF��NaClO2�����ݵ���غ��֪��NaF��NaCN����Һ�д���c(Na��)��c(H��)��c(OH��)��c(F��)��c(Na��)��c(H��)��c(OH��)��c(CN��)���������Һ�������������������ֱ���n(Na��)��n(H��)��n(OH��)��n(F��)��2 n(Na��)��2n(H��)��n(Na��)��n(H��)��n(OH��)��n(CN��)��2 n(Na��)��2n(H��)�������ʵ���Ũ����ȵ������£�NaF��Һ�ļ�������NaCN��Һ�ļ��ԣ���NaF��Һ�������ӵ�Ũ�ȴ���NaCN��Һ�������ӵ�Ũ�ȣ���˻���ȣ����ʵ���Ũ����ͬ��NaF��NaCN����Һ�������������������Ĵ�С��ϵΪǰ�ߴ�
�ڸ����ܶȻ�������֪��CuS���ܶȻ�������С���������е�Ũ�ȵ�Cu2+��Fe2+��Pb2+���ӵ���Һ�У��μ�Na2S��Һ�����������ij�����CuS�������������FeS����������Һ��c(Fe2+)��10-5mol/Lʱ������FeS���ܶȻ�������֪����Һ��c(S2��)��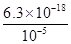 ��6.3��10��13mol/L��
��6.3��10��13mol/L��
���㣺����������ԭ��Ӧ����ʽ����ƽ�����㣻���ʵķ������ᴿ��������ʵĵ��롢����ˮ���Լ���Һ������Ũ�ȴ�С�Ƚϣ��ܶȻ�������Ӧ�úͼ����

 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д�(14��) CuCl���л��ϳɵ���Ҫ���������������ϡ������ȹ�ҵ����ҵ���ɷ�ͭ�ϣ���Fe��Al���仯���SiO2���ʣ�������CuCl�Ĺ����������£�
| ���� | ��ʼ���� | ������ȫ |
| Fe(OH)3 | 2.7 | 3.7 |
| Cu(OH)2 | 5.6 | 6.7 |
| Al(OH)3 | 3.8 | 4.7 |
��֪��CuCl����NaCl��Ũ��Һ������CuCl2����CuCl2������Һ��ˮϡ�ͺ������CuCl������
��1�����յ���ҪĿ������ ��
��2��������Ϊ������Һ��PHֵ����ΧΪ �����������X�����ǣ� ��
A��CuO B��Cu(OH)2 C��NaOH ��Һ D��CaCO3
��3�����������Ҫ�ɷ��� ��
��4������Һ���м���ʳ�β�ͨ��SO2������CuCl2������д����Ӧ�����ӷ���ʽ�� ��
��5���ڷ�Ӧ���У��¶ȿ�����70~80�沢ʹ��ŨNaCl��Һ����ҪĿ���ǣ� ��
��6�������£���֪CuOH��KSPΪ1.0��10��14����Cu++H2O
 CuOH + H+��ƽ�ⳣ��Ϊ�� ��
CuOH + H+��ƽ�ⳣ��Ϊ�� �� 
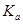 =____________mol/L��
=____________mol/L�� Fe(OH)3+3HC1;��H��0���ش��������⣺
Fe(OH)3+3HC1;��H��0���ش��������⣺