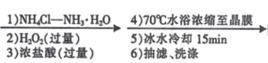
��Ŀ����
����Ŀ���ѡ�������������ͭ�Ƚ������仯�����ڹ�ҵ�϶�����Ҫ��;��
��1����̬��ԭ����Χ���ӵĹ������ʽΪ_____������ͬ���ڵ�Ԫ���У���̬ԭ�ӵ�δ�ɶԵ���������ԭ����ͬ����_____�֡�
��2��TiCl4���Ȼ�����ȡ�ѵ��м���TiCl4��SiC14�ڳ����¶���Һ�壬���ӽṹ��ͬ����������ķ�������TiCl4��SiCl4�Ļ����Ȼ�õ������_____���ѧʽ����
��3��[Cr��C2O4��2��H2O2��]Ҳ�Ǹ���һ�ֻ�����û������������ӻ�������г������Ӽ������ۼ��⣬������_____����
��4����ص�Σ�Reinecke salt���Ļ�ѧʽΪNH4[Cr��NCS��4��NH3��2] H2O����һ��������ˮ���Ҵ��İ���ɫ���塣
���������и�Ԫ�صĻ��ϼ�Ϊ_____��
��NCS�����幹����_____������̼ԭ�ӵ��ӻ��������Ϊ_____��
��SO2��CO2��BeCl2��SCl2���ַ����У���NCS-��Ϊ�ȵ��������_____���ѧʽ����
��5��Ni��La�ĺϽ���Ŀǰʹ�ù㷺�Ĵ�����ϣ��������������������͵��µ��ص㣬���ձ����й���ʵ���˲�ҵ�����úϽ�ľ����ṹ��ͼ��ʾ��
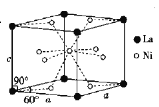
�پ���Ļ�ѧʽΪ_____��
�ڸþ������ܶ�Ϊdgcm-3����NAΪ�����ӵ�������ֵ����þ����������____���ú�d��NA�Ĵ���ʽ��ʾ��cm3��
�۸þ�����ڲ����п�϶����ÿ�������Ŀ�϶�д���6����ԭ��ʱ�Ƚ��ȶ�����֪: a=511pm,c=397pm;��״���£��������ܶ�Ϊ8.98X10-5 g�� ![]() ������������ǰ��������仯����ô�����ϵĴ�������Ϊ_____��������������
������������ǰ��������仯����ô�����ϵĴ�������Ϊ_____��������������
���𰸡�![]() 3 SiCl4 ��λ +3 ֱ���� sp�ӻ� CO2��BeCl2 ��LaNi5
3 SiCl4 ��λ +3 ֱ���� sp�ӻ� CO2��BeCl2 ��LaNi5 ![]() 1236
1236
��������
��1������Tiԭ����Χ�����Ų�ʽ3d24s2������Χ���ӵĹ������ʽΪ��![]() ��Tiԭ��δ�ɶԵ�����Ϊ2���ڵ�������Ԫ���У���̬ԭ�ӵ�δ�ɶԵ�����Ϊ2����Ni��3d84s2����Ge��4s24p2����Se��4s24p4�����ʴ�Ϊ��
��Tiԭ��δ�ɶԵ�����Ϊ2���ڵ�������Ԫ���У���̬ԭ�ӵ�δ�ɶԵ�����Ϊ2����Ni��3d84s2����Ge��4s24p2����Se��4s24p4�����ʴ�Ϊ��![]() ��3��
��3��
��2��TiCl4��SiC14�ڳ����¶���Һ�壬��֪���߾����ڷ��Ӿ��壬�ҷ��ӽṹ��ͬ����Է�������Խ���Ӽ�������Խ��TiCl4�ķе�SiC14�ߣ���������ķ�������TiCl4��SiCl4�Ļ����Ȼ�õ������SiCl4���ʴ�Ϊ��SiCl4��
��3��[Cr��C2O4��2��H2O2��]Ϊ�������п϶���������λ�����ʴ�Ϊ����λ��
��4�����������и�Ԫ�صĻ��ϼ�Ϊ+3���ʴ�Ϊ��+3��
���������εĽṹʽ��֪��NCS-������ԭ��Cԭ���γ�������˫����ÿ��˫������һ���Ҽ���һ���м������������幹��Ϊֱ���ͣ�����̼ԭ�ӵ��ӻ��������Ϊsp�ӻ����ʴ�Ϊ��ֱ���ͣ�sp�ӻ���
��NCS-����3��ԭ�ӣ��۵�����Ϊ16����SO2��CO2��BeCl2��SCl2���ַ����У���NCS-��Ϊ�ȵ�������У�CO2��BeCl2���ʴ�Ϊ��CO2��BeCl2��
��5���پ���������һ����ԭ�ӣ�����8����ԭ���ھ������ϣ���ԭ�Ӷ��ھ��������ϣ����Ծ���ʵ�ʺ��е���ԭ����Ϊ1+2��1/8=5����ԭ����Ϊ8��1/8=1�����Ծ����Ļ�ѧʽΪ��LaNi5���ʴ�Ϊ��LaNi5��
�ڸ���![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��LaNi5�Ͻ������ܶ�Ϊ��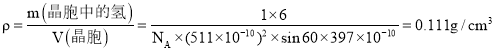 ���ɶ���ʽ
���ɶ���ʽ![]() ���ʴ�������=
���ʴ�������=![]() ���ʴ�Ϊ��1236��
���ʴ�Ϊ��1236��

 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д�