��Ŀ����
����Ŀ��ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ�������ָʾ��������д���пհף�
I.��1���ñ���������Һ�ζ����������������Һʱ,���ְ�����ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע��_________��ֱ�������һ���������Һ�ɻ�ɫ��Ϊ��ɫ��������ζ��յ㡣
��2�����в����п���ʹ��������������Һ��Ũ����ֵƫ�͵���_______________
A ��ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ
B �ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и���
C ��ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D ��ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
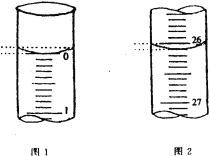
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ������ʼ����Ϊ_________mL���յ����Ϊ_____________mL������������Һ�����Ϊ______________mL��
��4��ijѧ����������ʵ��ֱ��¼�й��������±���
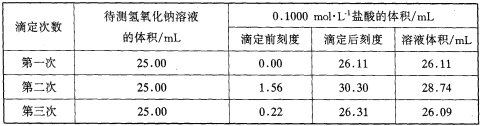
��ѡ�����к�����������ʽ���������������Һ�����ʵ���Ũ�ȣ�����4λС������c��NaOH��______
II.��H+Ũ����ͬ�������ȵ������a������b������ c�����ᣬͬʱ����������п����ʼ��Ӧʱ����_____________��Ӧ��ȫ������H2������ ___________����<��=��> ��ʾ��
���𰸡���ƿ����ɫ�仯 D 0.00mL 26.10mL 26.10mL 0.1044mol/L a=b=c a=b<c
��������
I.��1������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯��
��2��A����ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ����Һ��Ũ��ƫС�����V������ƫ����c�����⣩=![]() ����֪c������ƫ��ѡ��A����
����֪c������ƫ��ѡ��A����
B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и������Һ�����ʵ������䣬��V��������Ӱ�죬����c�����⣩=![]() ����֪c���������䣬ѡ��B����
����֪c���������䣬ѡ��B����
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V������ƫ����c�����⣩=![]() ����֪c������ƫ��ѡ��C����
����֪c������ƫ��ѡ��C����
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V������ƫС������c�����⣩=![]() ����֪c������ƫС��ѡ��D��ȷ��
����֪c������ƫС��ѡ��D��ȷ��
��ѡD��
��3����ʼ����Ϊ0.00mL���յ����Ϊ26.10mL��������Һ�����Ϊ26.10mL��
�������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ=26.10mL��
HCl+NaOH=NaCl+H2O
0.0261L��0.1000mol/L 0.025L��c��NaOH��
��c��NaOH��=![]() =0.1044mol/L��
=0.1044mol/L��
II.H+Ũ�ȴ�СӰ�����������ķ�Ӧ���ʣ�H+Ũ����ͬ����ʼʱ�ķ�Ӧ������ͬ����a=b=c��
����Ϊ���ᣬ������ȫ���룬��H+Ũ����ͬʱ�����Ũ�������������ᶼΪǿ�ᣬH+Ũ����ͬ����Һ�����ͬ����H+���ʵ�����ͬ������ȫ��Ӧ������H2������ɴ�С��c��a=b��

 ��У����ϵ�д�
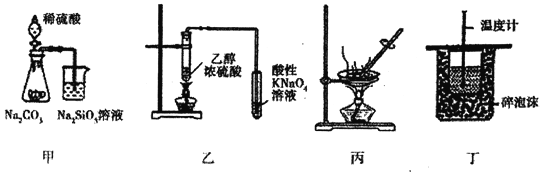
��У����ϵ�д�����Ŀ�������й����ʵ��Ʊ�װ�á��Ʊ�ʱʹ�õ�ԭ�����ռ���������ȷ���ǣ� ��
��� | ���� | �Ʊ�װ�� | �Ʊ�ʱʹ�õ�ԭ�� | �ռ����� | ��� | ���� | �Ʊ�װ�� | �Ʊ�ʱʹ�õ�ԭ�� | �ռ����� |
A |
|
|
| ��ˮ�� | B | NO |
| Ũ����ͽ���ͭ | �ſ����� |
C |
|
| Ũ��ˮ���������ƹ��� | �ſ����� | D |
|
| �Ҵ��� | ��ˮ�� |
A.AB.BC.CD.D