��Ŀ����
����Ŀ����֪��25��ʱ��0.1mol��L-lCH3COOH�ĵ���ȣ��ѵ����CH3COOH������/ԭCH3COOH����������ԼΪ1%�����¶��£���0.1000mol��L-l��ˮ�ζ�10.00 mL0.1000mol��L-lCH3COOH��Һ����Һ��pH����Һ�ĵ���������I���Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
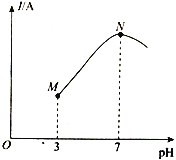
A. M���N�㣬ˮ�ĵ���̶�������
B. 25��ʱ��CH3COOH �ĵ��볣��ԼΪ1.0��10-2
C. N����Һ�У�c(CH3COO-) =c(NH4+)=0.05 mol��L-l
D. ������20 mL��ˮʱ����Һ��c(CH3COO-)>c(NH4+)
���𰸡�A
��������A��M���N�㣬M����Ҫ�Ǵ��ᣬ����ˮ���룬N����Ҫ�Ǵ���泥��ٽ�ˮ���룬ˮ�ĵ���̶�������A��ȷ��B��25��ʱ��CH3COOH �ĵ��볣��ԼΪk=0.1*1%*0.1*1%=1.0��10-6,��B����C��N����Һ�У�CH3COO-��NH4+����Ҫˮ�⣬��pHΪ7ʱ��c(CH3COO-) =c(NH4+)<0.05 mol��L-l����C����D��������20 mL��ˮʱ����Һ������Ϊ����狀Ͱ�ˮ������c(CH3COO-)+c(OH+)=c(NH4+)+c(H+),��Һ�ʼ��ԣ�����c(CH3COO-)<c(NH4+)����D����ѡA��

 �����ҵ���������ϵ�д�
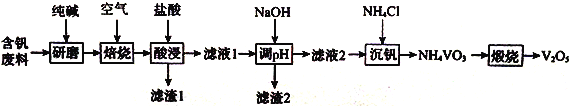
�����ҵ���������ϵ�д�����Ŀ��ij��ѧС���������������������װ��������ͼ��ʾ�����û������Ʊ�����ϩ��

��֪��
��Է������� | �ܶ�/g cm-3 | �۵�/�� | �е�/�� | �ܽ��� | |
������ | 100 | 0.96 | 25 | 161 | ������ˮ |
����ϩ | 82 | 0.81 | -103 | 83 | ������ˮ |
��1���Ʊ���Ʒ
��12.5 mL��������1mLŨ��������Թ�A�У�ҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
�����Թ��л�ϻ��Ѵ���Ũ�������ʱ������ҩƷ���Ⱥ�˳��Ϊ_________��
���������һ��ʱ��������Ǽ����Ƭ��Ӧ�ò�ȡ����ȷ������_________������ĸ����
A.�������� B.��ȴ�� C.���貹�� D.��������
�����Թ�C���ڱ�ˮ�е�Ŀ����_______________________________��
��2���Ʊ���Ʒ
������ϩ��Ʒ�к��л������������������ʵȡ����Ʒ�м��뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��__________________��(��ϡ����¡�)����Һ����__________________������ĸ��ϴ����
a.����KMnO4��Һ b.ϡ���� c.Na2CO3��Һ
���ٽ��ᴿ��Ļ���ϩ����ͼ��ʾװ�ý�������ͼ������a ��������_______________��ʵ������ȴˮ��__________������ĸ���ڽ��롣����ʱҪ������ʯ�ң�Ŀ����__________________________��

��3�������Ʊ���Ʒʱ���Ѵ����Ʒһ����������ʵ���ƵõĻ���ϩ��Ʒ����__________(�������������� ������)���۲�������ʵ�����õ��Ļ���ϩ����Ϊ6.25g���������___________________��