��Ŀ����
18�����������䡱�������������ص㹤�̣����������ָ��Ȼ��������Ҫ�ɷ��Ǽ��飬��ҵ�Ͻ�̼��ˮ�ڸ����·�Ӧ�Ƶ�ˮú����ˮú������Ҫ�ɷ���CO��H2�����ߵ������ԼΪ1��1����֪1mol CO������ȫȼ������CO2����ų�283kJ������1mol H2��ȫȼ������Һ̬ˮ�ų�286kJ������1mol CH4������ȫȼ������CO2�����Һ̬ˮ�ų�890kJ��������1��д��H2��ȫȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��2H2��g��+O2��g��=2H2O��l����H=-572kJ•mol-1����1mol CH4������ȫȼ������CO2�����ˮ�������ų���������890kJ���������=����������
��2������ˮú���������ɷ֣���ͬ״�������õ���ȵ�����������ˮú�������������ԼΪ3.1��1��ȼ�����ɵ�CO2��������ԼΪ1.55��1��
���� ��1������Ȼ�ѧ����ʽ��д��������ע���ʾۼ�״̬�Ͷ�Ӧ�ʱ䣻Һ̬ˮ��Ϊ��̬ˮ���ȣ�
��2��ˮú������Ҫ�ɷ���CO��H2�����ߵ������Ϊl��l��l mol CH4������ȫȼ������CO2�������̬ˮ�ų�802kJ����������Ȼ�ѧ����ʽ��������֮�ȣ�
��� �⣺��1��1mol H2��ȫȼ������Һ̬ˮ�ų�286kJ����������H2��ȫȼ��������̬ˮ���Ȼ�ѧ����ʽ��2H2��g��+O2��g��=2H2O��l����H=-572kJ•mol-1��1mol CH4������ȫȼ������CO2�����Һ̬ˮ�ų�890kJ������Һ̬ˮ��Ϊ��̬ˮ���ȣ�������ͬ�����£�1mol CH4������ȫȼ������CO2�����ˮ�������ų�������С��890kJ��
�ʴ�Ϊ��2H2��g��+O2��g��=2H2O��l����H=-572kJ•mol-1������
��2�������Ȼ�ѧ����ʽ��2H2��g��+O2��g��=2H2O��l����H=-572kJ•mol-1��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890kJ/mol��2CO��g��+O2��g��=2CO2��g����H=-566KJ/mol��ˮú������Ҫ�ɷ���CO��H2�����ߵ������Ϊl��l��1molCH4ȼ�շ���890kJ��1molCO��H2����������$\frac{283+286}{2}$kJ=284.5kJ����ͬ״�������õ���ȵ�����������ˮú�������������ԼΪ$\frac{Q}{284.5}$��$\frac{Q}{890}$=3.1��1��ȼ�����ɵ�CO2��������ԼΪ$\frac{3.1��\frac{1}{2}��44}{1��44}$=1.55��1��
�ʴ�Ϊ��3.1��1��1.55��1��
���� ������Ҫ�������Ȼ�ѧ����ʽ��д����Ӧ�����ļ���Ӧ�ã�ȼ�������ıȽϷ�����ע�ⷴӦ�е����������ʵ��������ȣ���Ŀ�Ѷ��еȣ�

| A�� | �þƾ���ȡ��ˮ�еĵ� | |
| B�� | ����ʱ����ȴˮ�������ܵ��Ͽ�ͨ�룬�¿����� | |
| C�� | �÷�Һ©����Һʱ���²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� | |
| D�� | ����1.00 mol/L NaCl��Һʱ�����ƺõ�NaCl����ֱ�ӷ�������ƿ���ܽ� |
| A�� | ����-�⻯����Һ����ˮ | B�� | ����������Һ���ˮ | ||
| C�� | ����Cu��OH��2����Һ���ˮ | D�� | �⻯����Һ���ˮ |
| A�� | ��ϩ�ı���ģ�ͣ� | |
| B�� | ���ĵ���ʽ��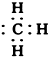 | |
| C�� | �Ҵ������ʽ��C2H6O | |
| D�� | ������Ϊ53��������Ϊ78�ĵ�ԭ�ӣ�${\;}_{53}^{111}$I |
| A�� | �ò�˿պȡNaC1��Һ�ھƾ��ƻ��������գ�����ʻ�ɫ | |
| B�� | SO2ʹƷ����Һ��ɫ | |
| C�� | ��ʳ����ϴ��ˮƿ�ڵ�ˮ�� | |
| D�� | ��װ��Fe��NO3��2��Һ���Թ��еμ�ϡH2SO4�����Թܿڹ۲쵽����ɫ���� |
| A�� | ��������Һ�м�������İ�ˮ��Al3++3NH3•H2O�TAl��OH��3��+3NH4+ | |
| B�� | �Ȼ�þ��Һ�м�������������Һ��Mg2++2OH-�TMg��OH��2�� | |
| C�� | ����ʯ���ڴ����У�CaCO3+2CH3COOH�TCa2++2CH3COO-+CO2��+H2O | |
| D�� | NH4HCO3���ڹ�����NaOH��Һ�У�HCO3-+OH-�TCO32-+H2O |

 ��
��