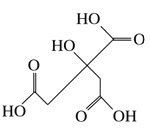
��Ŀ����
����Ŀ��ʵ��������Ҫ������NaCl���壬������ֻ�л���Na2SO4��NH4HCO3��NaCl�������ijѧ���������ͼ��ʾ������ȡ������NaCl���塣(��֪��NH4HCO3![]() NH3����CO2����H2O)
NH3����CO2����H2O)

����˷�����ȷ���ش��������⣺
(1)�����ٿ�ѡ�����Ҫ��������________��(��ѡ��)
A.�ƾ��� B. ������ƿ C. ����
(2)�����ڲ������ᱵ��Һ����������______________________________________��
(3)���в����ں�����ж�SO42 �ѳ�����������________________________��
(4)�����ۼӵ�����________��
A.Na2CO3��Һ B.K2CO3��Һ C. NaNO3��Һ
(5)�����ܵ�Ŀ������________��(��ѡ��)
A����ȥ������BaCl2��Һ
B.��ȥ�ܽ�����Һ�е�CO2
C. ��ȥ�ܽ�����Һ�е�HCl
���𰸡�AC ��Һ�������µ���������NO3- ȡ�����ϲ���Һ����BaCl2��Һ�����ް�ɫ����˵��SO42-�ѳ��� A BC
��������
��ʵ�������ʵķ�����ᴿʵ�飬NH4HCO3���ȷֽ⣬Na2SO4��NaCl����ʱ���ȶ������ȿɳ�ȥNH4HCO3��Na2SO4��NaCl��������ˮ���γ���Һ��ӿ����Ա��γ�ȥSO42-��Ϊ�������µ�������Ҫѡ����ʵ��Լ��������ij����Լ�ҲҪ��ȥ��NaCl���ܽ�����¶ȵ������������ԣ�ͨ������NaCl��Һʹ��ᾧ�õ�������NaCl��
(1)��NH4HCO3�����ֽ�NH4HCO3![]() NH3
NH3![]() +CO2
+CO2![]() +H2O���ҷֽ��û�й���������NaCl��Na2SO4���Ȳ��ֽ⣬�ü��ȵķ�����ȥNH4HCO3�����ȹ���ʱͨ�������������������þƾ��Ƽ��ȣ���ѡAC��
+H2O���ҷֽ��û�й���������NaCl��Na2SO4���Ȳ��ֽ⣬�ü��ȵķ�����ȥNH4HCO3�����ȹ���ʱͨ�������������������þƾ��Ƽ��ȣ���ѡAC��
(2)�����ڵ�Ŀ���ǽ�Na2SO4��NaCl�����Һ�е�SO42-��ȥ��ͨ��ѡ������Ժ���������������ӷ�ӦSO42-+Ba2+=BaSO4����SO42-ת��Ϊ���������˶���ȥ����Ϊ�˲������µ����ʣ�������������Ӧ��Cl-�����Բ���ѡ�����ᱵ��Һ�������к�NO3-�������µ����ʡ�
(3)���в����ں���Һ�м�������SO42-���������Ƿ���ڼ����ж�SO42-�Ƿ�������������ӷ�ӦSO42-+Ba2+=BaSO4��������SO42-���䷽���ǣ�ȡ�����ϲ���Һ����BaCl2��Һ�����ް�ɫ����˵��SO42-�ѳ�����
(4)Ϊ�˳���SO42-�������������ӵ�BaCl2��ҺҪ������������BaCl2����ҲҪ��ȥ�������۵�Ŀ�ľ��dz�ȥ�����Ba2+��������CO32-+Ba2+=BaCO3���ٹ��˳�ȥBaCO3���ɣ�Ϊ�˲������µ�����ֻ��ѡ��Na2CO3��Һ����ѡA��
(5)������������������Ϊ�˳����������й�����CO32-��CO32-+2H+=CO2��+H2O�������ɵ�CO2������ˮ�������Һ����������HCl��CO2���ʣ���HCl��CO2�����лӷ��ԣ����Լ�����м��ɳ���HCl��CO2����ѡBC��