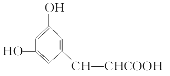
��Ŀ����
����Ŀ��ʵ����
ʵ������ȡ��ϩ�����Ҵ���Ũ���ᷴӦ�������¶ȹ��߶���������Ӧ����̿�ڡ�������������̼��ˮ������������ͼ���Ϊ��~��ʵ��װ�ã����һ��ʵ�飬����֤������Ӧ��Ļ�������к��ж�����������̼��ˮ������

��1����װ�õı�ű�ʾ������˳�������������ҵ�����6������ ������3������ ������ ������ ��____
��2��װ�â��еĹ���ҩƷΪ____��������֤�IJ�����____��
��3��װ�â��е���Һ��____��������֤�IJ�����____��
��4��װ�â��е�Ʒ����Һ������_____��
��5��װ�â�����ˮ��������____��д�����п��ܷ�����2����ѧ����ʽ____��____��
���𰸡���6������5������3������2������1����4�� ��ˮ����ͭ��ĩ H2O ����ʯ��ˮ CO2 ����SO2�Ƿ��� ����CO2�е�SO2 SO2+Br2+2H2O=H2SO4+2HBr CH2=CH2+Br2��BrCH2CH2Br
��������
(1)�������������Ʒ����Һ�����������̼�ó����ʯ��ˮ������ˮ��������ˮ����ͭ������Һ����ˮ���������ȼ���ˮ��������ΪCO2���ó���ʯ��ˮ������ģ���SO2Ҳ����ʹ����ʯ��ˮ��룬����������ʹƷ����ɫ��������̼���ܣ����ԣ�������������ڼ��������̼֮ǰ����ͨ������ʯ��ˮȷ��CO2���ڣ��ݴ�����װ�ã�
(2)��ɫ����ˮ����ͭ��ˮ��Ӧ������ɫ����ͭ���壬�ݴ˼�����ˮ���ɣ�
(3)������̼�ܹ�ʹ����ʯ��ˮ����ǣ��ݴ˼��������̼����Ĵ��ڣ�
(4)װ��(1)Ʒ����ɫ��˵���ж������������
(5)��ˮ���ڳ�ȥ��������еĶ�����������������ˮ��Ӧ����������廯�⣬�ݴ�д����Ӧ�Ļ�ѧ����ʽ��
(1)ʵ��������ϩѡ��װ��(6)��ͬʱ�Ҵ���ŨH2SO4�¶ȹ��ᷢ����Ӧ��CH3CH2OH+4H2SO4(Ũ)![]() 4SO2��+CO2��+7H2O+C������Һ����ˮ�����Ա����ȼ���ˮ������ѡ��װ��(5)��(5)��ʢ����ˮ����ͭ��������˵����ˮ���ɣ��������̼�Ͷ���������ʹ�����ʯ��ˮ����ǣ�����������ʹƷ����ɫ��������̼���ܣ�������ͨ��ʢ��Ʒ����Һ��װ��(3)��Ʒ����Һ��ɫ��˵����SO2���������(2)װ������SO2��(1)װ��Ʒ�첻��ɫ��˵�����������Ѿ�������ȫ�������װ��(4)��ͨ������ʯ��ˮ�����ȷ��CO2���ڣ�����װ�õ�����˳��Ϊ��(6)(5)(3)(2)(1)(4)��
4SO2��+CO2��+7H2O+C������Һ����ˮ�����Ա����ȼ���ˮ������ѡ��װ��(5)��(5)��ʢ����ˮ����ͭ��������˵����ˮ���ɣ��������̼�Ͷ���������ʹ�����ʯ��ˮ����ǣ�����������ʹƷ����ɫ��������̼���ܣ�������ͨ��ʢ��Ʒ����Һ��װ��(3)��Ʒ����Һ��ɫ��˵����SO2���������(2)װ������SO2��(1)װ��Ʒ�첻��ɫ��˵�����������Ѿ�������ȫ�������װ��(4)��ͨ������ʯ��ˮ�����ȷ��CO2���ڣ�����װ�õ�����˳��Ϊ��(6)(5)(3)(2)(1)(4)��
(2)װ��(5)�������Ǽ���ˮ�������ð�ɫ����ˮ����ͭ��ĩ��CuSO4+5H2O�TCuSO45H2O����˵�������������ˮ������
(3)��װ��(4)�������������̼��������̼��ʹ����ʯ��ˮ����ǣ�����װ��(4)��ʢ�г���ʯ��ˮ�����ڼ���CO2��
(4)ʵ��ʱװ��(1)�������Ǽ����������Ĵ��ڣ��ж�װ��(2)��û����ȫ����SO2��ƿ��װ��Ʒ����Һ������������ʹƷ����ɫ������(1)��Ŀ���Ǽ���SO2�Ƿ�����
(5)��ϩ�Ͷ���������ʹ��ˮ��������������Һ��ɫ����������Ĵ���Ӱ����ϩ�ļ��飬�ʼ�����ϩʱӦ�ȳ�ȥ��������װ��(2)����ˮ���ڳ�ȥ��������Ӧ�Ļ�ѧ����ʽΪ��SO2+Br2+2H2O�TH2SO4+2HBr�����������������ϩ�����ӳɷ�Ӧ����Ӧ����ʽΪCH2=CH2+Br2��BrCH2CH2Br��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��I.��1�� SiH4��һ����ɫ���壬���������ܷ�����ը����ȼ������SiO2(s)��H2O(l)����֪������2 g SiH4��ȼ�ų�������Ϊ89.2 kJ�������Ȼ�ѧ����ʽΪ�� _____________________��
��2��������һ����Դ��������Ҫ�ɷ���CH4����ȼ�յ��Ȼ�ѧ����ʽΪ��CH4(g)��2O2(g) = CO2(g)��2H2O(l )����H����890 kJ/mol������֪H2O(l)=H2O(g)����H����44 kJ/mol����11.2 L(��״��)CH4��ȫȼ��������̬ˮʱ�ų�������Ϊ_______________��
��.��֪���м���������ȼ�������£�
���� | ���� | ���� | ���� | ���� | ���� | ���� |
ȼ����/ (kJ��mol��1) | 890.3 | 1559.8 | 2219.9 | 2877.0 | 3536.2 | 4163.1 |
����10 L(��״����)ij����Ȼ���������������������������壬ȼ��ʱ���ų�����480 kJ��
��1����д����������ȼ�յ��Ȼ�ѧ����ʽ___________________________________��
��2���������Ȼ���м�����������____________(����С�������λ)��
��3�����ϱ����ܽ���Ľ��ƹ�����______________________________________________��
��4�����ݣ�3���Ľ��ƹ��ɿ�Ԥ������ȼ����ԼΪ______kJ��mol��1��