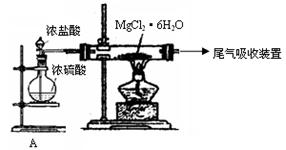
��Ŀ����
��19�֣�ij����A����ͼ��ʾ�Ĺ���ת��Ϊ������D����֪DΪǿ�ᣬ��ش��������⣺

��1����A������Ϊ����ɫ���嵥�ʣ�B���д̼�����ζ����ɫ���塣
�����A��Ԫ�������ڱ�λ��Ϊ������������������������
��Ӧ���ӵĽṹʾ��ͼΪ����������������������������������
��д��B��C��Ӧ�Ļ�ѧ����ʽ������������������������������������
������Bͨ��Ʒ����Һ�У���������������������������������B��
�ԣ�����Bͨ����ɫ��ʯ����Һ�У���������������������������������������
������B��ˮ��Һ�� �ԡ�
��D��Ũ��Һ�ڼ��������¿���ͭ��Ӧ������B���壬�÷�Ӧ�Ļ�ѧ����ʽΪ��
��������������������������������������
��2����A�ڳ�����Ϊ���壬����ʹʪ��ĺ�ɫʯ����ֽ������C�Ǻ���ɫ���塣
��A��C�Ļ�ѧʽ�ֱ��ǣ�A����������������C
��д��A��B��Ӧ�Ļ�ѧ����ʽ��������������������������������
������Aͨ����з�̪��ˮ�У������������� ���� ����ʽ��ʾ��ԭ��
����ʽ��ʾ��ԭ��
��
��D��Ũ��Һ����ͭ��Ӧ������C���壬��÷�Ӧ�Ļ�ѧ����ʽΪ��
��������������������������������������������������

��1����A������Ϊ����ɫ���嵥�ʣ�B���д̼�����ζ����ɫ���塣
�����A��Ԫ�������ڱ�λ��Ϊ������������������������
��Ӧ���ӵĽṹʾ��ͼΪ����������������������������������
��д��B��C��Ӧ�Ļ�ѧ����ʽ������������������������������������
������Bͨ��Ʒ����Һ�У���������������������������������B��
�ԣ�����Bͨ����ɫ��ʯ����Һ�У���������������������������������������
������B��ˮ��Һ�� �ԡ�
��D��Ũ��Һ�ڼ��������¿���ͭ��Ӧ������B���壬�÷�Ӧ�Ļ�ѧ����ʽΪ��
��������������������������������������
��2����A�ڳ�����Ϊ���壬����ʹʪ��ĺ�ɫʯ����ֽ������C�Ǻ���ɫ���塣
��A��C�Ļ�ѧʽ�ֱ��ǣ�A����������������C
��д��A��B��Ӧ�Ļ�ѧ����ʽ��������������������������������
������Aͨ����з�̪��ˮ�У������������� ����
 ����ʽ��ʾ��ԭ��
����ʽ��ʾ��ԭ����
��D��Ũ��Һ����ͭ��Ӧ������C���壬��÷�Ӧ�Ļ�ѧ����ʽΪ��
��������������������������������������������������
(��19��)��2���١�����������VIA�塡���� ���� (��1��)
���� (��1��)
�ڡ�2SO2+O2 2SO3(2��)
2SO3(2��)
�����ۡ�Ʒ����Һ��ɫ��Ư�ף���Һ��죻��(��1��)
�ܡ�Cu+2 H2SO4��Ũ����Cu SO4 +SO2��+2H2O (2��)
����2���١�NH3�� NO2(��1��) ��4NH3+5O2== 4NO+6H2O (2��)
��������Һ���(1��)����NH3+H2O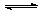 NH3�� H2O
NH3�� H2O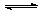 NH4++OH- (2��)
NH4++OH- (2��)
��Cu��4HNO3(Ũ)��Cu(NO3)2��2NO2����2H2O (2��)
 ���� (��1��)
���� (��1��)�ڡ�2SO2+O2
 2SO3(2��)
2SO3(2��)�����ۡ�Ʒ����Һ��ɫ��Ư�ף���Һ��죻��(��1��)
�ܡ�Cu+2 H2SO4��Ũ����Cu SO4 +SO2��+2H2O (2��)
����2���١�NH3�� NO2(��1��) ��4NH3+5O2== 4NO+6H2O (2��)
��������Һ���(1��)����NH3+H2O
 NH3�� H2O
NH3�� H2O NH4++OH- (2��)
NH4++OH- (2��)��Cu��4HNO3(Ũ)��Cu(NO3)2��2NO2����2H2O (2��)
��

��ϰ��ϵ�д�
�����Ŀ
 (1)Ԫ��Q��Ԫ�����ڱ��е�λ��______________________________��
(1)Ԫ��Q��Ԫ�����ڱ��е�λ��______________________________��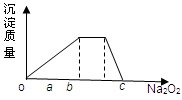
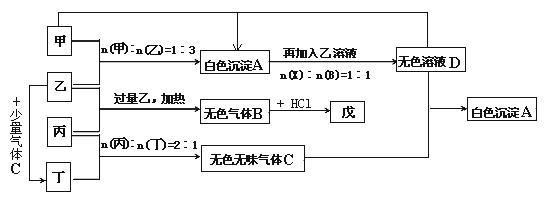
 ����________�壻
����________�壻 ) д��ͭ��ϡHBO3��Һ��Ӧ�����ӷ�Ӧ����ʽ
) д��ͭ��ϡHBO3��Һ��Ӧ�����ӷ�Ӧ����ʽ 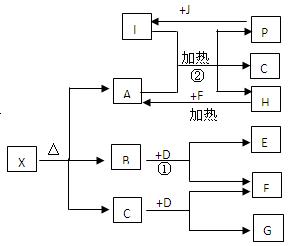
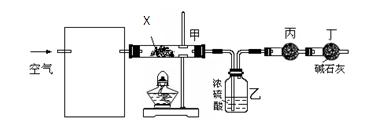
 þ����Ҫ�������£�
þ����Ҫ�������£�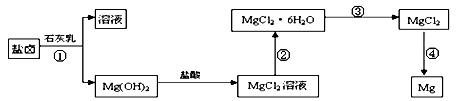
 �ش��������⣺
�ش��������⣺ ��
��