��Ŀ����
8����֪��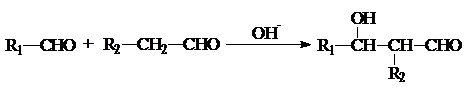 ���л���G��C20H18O4����һ�������ϳ�·�����£�
���л���G��C20H18O4����һ�������ϳ�·�����£�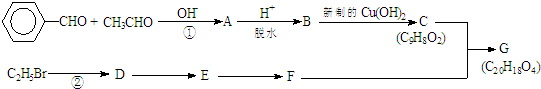
�Իش��������⣺
��1��ָ����Ӧ���ͣ���Ӧ�ټӳɣ���Ӧ����ȥ��
��2��A�Ľṹ��ʽ��
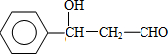 ��F �Ľṹ��ʽ��CH2OHCH2OH��
��F �Ľṹ��ʽ��CH2OHCH2OH����3��C��Fת��ΪG�Ļ�ѧ����ʽ�ǣ��л����ýṹ��ʽ��ʾ����
 +CH2OHCH2OH$��_{��}^{Ũ����}$
+CH2OHCH2OH$��_{��}^{Ũ����}$ +2H2O��
+2H2O����4��ʵ��Eת��ΪF�ķ�Ӧ����Ϊ�������Ƶ�ˮ��Һ�����ȣ�
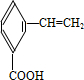
��5���л���C��ͬ���칹�壨��C�⣩��ͬʱ����̼̼˫�����������Ȼ����У�

��
 ��
�� ��
�� ��
��
���� �������Ϣ��֪AΪ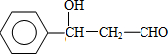 ����ˮ������BΪ
����ˮ������BΪ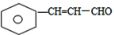 ��B�����Ʊ�������ͭ��Ӧ����CΪ
��B�����Ʊ�������ͭ��Ӧ����CΪ ����C��G�ķ���ʽ��֪FӦΪCH2OHCH2OH����DΪCH2=CH2��EΪCH2BrCH2Br����������Ϣ���л���Ľṹ�����ʽ����⣮
����C��G�ķ���ʽ��֪FӦΪCH2OHCH2OH����DΪCH2=CH2��EΪCH2BrCH2Br����������Ϣ���л���Ľṹ�����ʽ����⣮
��� �⣺�������Ϣ��֪AΪ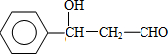 ����ˮ������BΪ
����ˮ������BΪ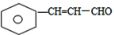 ��B�����Ʊ�������ͭ��Ӧ����CΪ
��B�����Ʊ�������ͭ��Ӧ����CΪ ����C��G�ķ���ʽ��֪FӦΪCH2OHCH2OH����DΪCH2=CH2��EΪCH2BrCH2Br��
����C��G�ķ���ʽ��֪FӦΪCH2OHCH2OH����DΪCH2=CH2��EΪCH2BrCH2Br��
��1����Ӧ����C=O���C-O����ӦΪ�ӳɷ�Ӧ��DΪCH2=CH2��Ӧ���ȴ���������ȥ��Ӧ���ɣ�
�ʴ�Ϊ���ӳɣ���ȥ��
��2�������Ϸ�����֪A�Ľṹ��ʽ��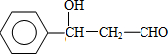 ��F�Ľṹ��ʽ��CH2OHCH2OH��
��F�Ľṹ��ʽ��CH2OHCH2OH��
�ʴ�Ϊ��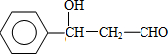 ��CH2OHCH2OH��
��CH2OHCH2OH��
��3�������Ϸ�����֪C�Ľṹ��ʽΪ ��F�Ľṹ��ʽΪCH2OHCH2OH������C��Fת��ΪG�Ļ�ѧ����ʽΪ
��F�Ľṹ��ʽΪCH2OHCH2OH������C��Fת��ΪG�Ļ�ѧ����ʽΪ +CH2OHCH2OH$��_{��}^{Ũ����}$
+CH2OHCH2OH$��_{��}^{Ũ����}$ +2H2O��
+2H2O��
�ʴ�Ϊ�� +CH2OHCH2OH$��_{��}^{Ũ����}$
+CH2OHCH2OH$��_{��}^{Ũ����}$ +2H2O��
+2H2O��
��4�������Ϸ�����֪EΪCH2BrCH2Br��FΪCH2OHCH2OH������Eת��ΪF�ķ�Ӧ����Ϊ�������Ƶ�ˮ��Һ�����ȣ��ʴ�Ϊ���������Ƶ�ˮ��Һ�����ȣ�
��5��CΪ ����Ӧ��ͬ���칹����ͬʱ��̼̼˫�����������Ȼ����У�
����Ӧ��ͬ���칹����ͬʱ��̼̼˫�����������Ȼ����У�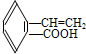 ��
�� ��
�� ��
�� �����֣�
�����֣�
�ʴ�Ϊ�� ��
�� ��
�� ��
��
���� ���⿼���л�����ƶϣ���Ŀ�ѶȽϴ���ȷ�л��������Ϊ������Ĺؼ���ע���������Ϣ��Ϸ�Ӧ�����й����ŵı仯�жϣ�

| A�� | �������� | B�� | ��ѩ�ڻ� | C�� | ʳ�︯�� | D�� | �·����� |
| A�� | ʳƷ�д������ӱ������Ƶȷ�����������Ч�ӳ��䱣���� | |
| B�� | ���ع��͡������Ƴɷ����������Դ�������� | |
| C�� | �ӿ쿪����Ч�ྻ��Դת��������������ԴΣ�� | |
| D�� | ��A PEC������2014���µ�����ʻ㣬����2014��A PEC�����ڼ䱱����������գ�˵������ʵʩ��·���к���Ⱦ��ҵͣ���ȴ�ʩ���Լ�����������֤������������Ч�� |
| ������� | HCOOH | HCN | H2CO3 | NH3•H2O |
| ����ƽ�ⳣ�� ��25�棩 | Ka=1.8��10-4 | Ka=4.9��10-10 | Ka1=4.3��10-7Ka2=5.6��10-11 | Kb=1.8��10-5 |
| A�� | ���H+��������CO32-��CN-��HCO3-��HCOO- | |
| B�� | 0.1mol/L��HCOONH4��Һ�У�c��HCOO-����c��NH4+����c��H+����c��OH-�� | |
| C�� | 25��ʱ��pH=3��������pH=11�İ�ˮ��ϣ�����Һ�����ԣ���������ĵ�����ǣ�V�����ᣩ��V����ˮ�� | |
| D�� | 0.1mol/L��NaHCO3��Һ�У�c��Na+��+c��H+��=c��HCO3-��+c��OH-��+c��CO32-�� |
| A�� | 7.8g�������ƺ��еĹ��õ��Ӷ���Ϊ0.2NA | |
| B�� | 2H2O2��l��=2H2O��l��+O2��g����H=-98.2 kJ •mol-1����S=70.5 J•mol-1•K-1���÷�Ӧ�������Է����� | |
| C�� | ��������ʹ���������Һ��ɫ��1mol��������õ�2NA���� | |
| D�� | �ں���NH4+��Ba2+��Cl-��NO3-���ӵ���Һ �������������������ϸ��������������� |
| A�� | ���ʱ���������Һ�е�H+�������ƶ� | |
| B�� | ���� 0.4 mol����ת�ƣ������� 2.24 L���� | |
| C�� | �����Ϸ�����ԭ��Ӧ�������Ϸ���������Ӧ | |
| D�� | �����ϵķ�ӦΪ��CH3CH2OH-4e-+H2O�TCH3COOH+4H+ |
| A�� | �ƹ�����̫���ܡ����ܵij�������ϵͳ����չ��̼���ú�ѭ�����ã������ڸ��ƻ������� | |
| B�� | ��֬�ڼ�������¿ɷ���ˮ�⣬��ҵ�����ø÷�Ӧ�������� | |
| C�� | ������ˮʱ�ɼ���������Ϊ��������������ˮ�е����� | |
| D�� | Ӳ����̼�ظֶ��ǺϽ���ϣ��ϳ���ά�����ά�����л��߷��ӻ����� |
| �� | ���� | ������ | ������ |
| ���볣����Ka�� | 1.7��10-5 | 3.0��10-8 | 7.1��10-4 |
| A�� | PH��ͬ����ˮ����������Һ��ˮ�������c��H+����ͬ | |
| B�� | ����ˮ�μ�NaOH��Һ�����ԣ�c��Na+��=2��ClO-��+c��HClO�� | |
| C�� | Ũ����ȵ�CH3COONa��NaNO2������Һ�У�c��CH3COO��-��c��NO${\;}_{2}^{-}$�� | |
| D�� | ��pH=a�Ĵ�����Һ�м�һ����ˮ��������Һ��pH��a��pH��a��pH=a���п��� |
| A�� | A��B��Ϊ���壬ƽ�������ƶ� | B�� | AΪ���壬ƽ�������ƶ� | ||
| C�� | BΪ���壬ƽ�ⲻ�ƶ� | D�� | A��B���������壬ƽ�������ƶ� |