��Ŀ����
17��25��ʱ�����ᡢ�����ᡢ������ĵ��볣�����±���������������ȷ���ǣ�������| �� | ���� | ������ | ������ |
| ���볣����Ka�� | 1.7��10-5 | 3.0��10-8 | 7.1��10-4 |
| A�� | PH��ͬ����ˮ����������Һ��ˮ�������c��H+����ͬ | |
| B�� | ����ˮ�μ�NaOH��Һ�����ԣ�c��Na+��=2��ClO-��+c��HClO�� | |
| C�� | Ũ����ȵ�CH3COONa��NaNO2������Һ�У�c��CH3COO��-��c��NO${\;}_{2}^{-}$�� | |
| D�� | ��pH=a�Ĵ�����Һ�м�һ����ˮ��������Һ��pH��a��pH��a��pH=a���п��� |
���� A��pH��ͬ�IJ�ͬ����Һ�У�����������������Ũ����ͬ��
B���ݵ���غ���c��Na+��+c��H+��=c��ClO-��+c��OH-�����������غ���c��Cl- ��=c��ClO-��+c��HClO�����ݴ˷�����
C����������Ա�������������Խ��Խˮ�������
D��������Һ��ˮϡ��ʱ�����Լ�����pH��С��
��� �⣺A��pH��ͬ�IJ�ͬ����Һ�У�����������������Ũ����ͬ����ˮ�ĵ�������Ƴ̶���ͬ������ˮ�������c��H+����ͬ����A��ȷ��
B�������Һ�����ԣ���c��H+��=c��OH-������Һ�д��ڵ���غ㣬����c��Na+��+c��H+��=c��OH-��+c��ClO-��+c��Cl-�������Ե�c��Na+��=c��ClO-��+c��Cl-�������������غ��c��Cl- ��=c��ClO-��+c��HClO�������Ե�c��Na+��=2c��ClO-��+c��HClO������B��ȷ��
C��Ũ����ȵ�CH3COONa��NaNO2������Һ�У���������Ա��������������������ӵĵ���̶ȴ�������������ӣ�����c��CH3COO-����c��NO2-������C����
D����pH=a�Ĵ�����Һ�м�һ����ˮ������ĵ���̶���������Һ��������Ũ�ȼ�С����Һ��pH����pH��a����D����
��ѡCD��
���� ���⿼����Ӱ��ˮ�ĵ�������ء���Һ�еĵ���غ�������غ㡢����ˮ�⡢�����ˮϡ��ʱ����Ũ�ȱ仯����Ŀ�ѶȲ���

| A�� | Al14��42���۵��ӣ�����IIA��Ԫ���������� | |
| B�� | Al13��39���۵��ӣ�����±���������� | |
| C�� | Al13����������HI��Ӧ������HAl13I���һ�ѧ����ʽΪ Al13+HI=HAl13I | |
| D�� | Al13ԭ����A1ԭ�Ӽ���ͨ�����Ӽ���ϵ� |
 �±��г��˶���ijЩ��ѧ�������������
�±��г��˶���ijЩ��ѧ�������������| ��ѧ�� | H-H | Cl-Cl | I-I | O�TO | C-Cl | C-H | O-H | H-Cl | H-I |
| ����1mol��ѧ�������յ�������kJ�� | 436 | 247 | 151 | x | 330 | 413 | 463 | 431 | 299 |
��1����ͼ��ʾij��Ӧ�������仯��ϵͼ�����������仯��ϵ ͼ��ʾ��ӦH2��g��+$\frac{1}{2}$O2��g���TH2O��g����
��H=-241��.8kJ•mol-1����B=926kJ•mol-1��x=496.4��
| A�� | �������������ᷴӦ��������������ɫͿ�� | |
| B�� | ���뺣�ڵĸ���բ����װһ��������ͭ��ɷ�ֹբ�ű���ʴ | |
| C�� | ��������������Ԫ�أ�����Ҫ��Ը����ߵ����ʳ�� | |
| D�� | ά����C��ˮ����ά���أ�����ǿ����ֿ������нⶾ���� |
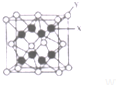 A��E����Ԫ�ؾ�λ�����ڱ���ǰ�����ڣ��й����ǵ���Ϣ���±���ʾ��
A��E����Ԫ�ؾ�λ�����ڱ���ǰ�����ڣ��й����ǵ���Ϣ���±���ʾ���ش���������
| Ԫ�� | �����Ϣ |
| A | Ԫ��A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�� |
| B | B�ĵ�����˫ԭ�ӷ��ӹ��ɣ���������14������ |
| C | C3�����ڴ������ƽ�����У���Ũ�ȵļ��ٻᵼ���˻�Ƥ���� |
| D | D��ǰ������Ԫ���е�һ��������С��Ԫ�� |
| E | Eλ��ds����ԭ�ӵ�������������A��ͬ |
��1��CԪ�ػ�̬ԭ�ӵļ۵����Ų�ͼΪ

��2��[E��A2C��4]2+��E2+��A2C�������ΪB������ĸ���
A�����Ӽ� B����λ�� C�������� D�����Ӽ�������
��3��D2C�ľ����ṹ��CaF2��������ͼ�����ƣ���XӦΪK+
D2C���۵��CaF2���۵�ͣ���ߡ������͡��������Ƚϡ���
��4����B2C��Ϊ�ȵ��������ΪCO2������ӵ����幹��Ϊֱ����
��5����֪��A2��g��+$\frac{1}{2}$C2��g��=A2C��g����H1=-akJ/mol
B2��g��+3A2��g��=2BA3��g����H2=-bkJ/mol
��34gBA3��g����C2��g����Ӧ����B2��g����A2C��g��ʱ���÷�Ӧ���Ȼ�ѧ��Ӧ����ʽΪ2NH3��g��+$\frac{3}{2}$O2��g��=N2��g��+3H2O��g����H=��b-3a��kJ/mol��
| ��� | ʵ��Ŀ�� | ʵ����� |
| A | ֤��CH2�TCHCHO�к���̼̼˫�� | ����KMnO4������Һ�����Ϻ�ɫ�Ƿ���ȥ |
| B | ����̼������Һ��̼��������Һ | �ֱ��������������������Һ���۲����� |
| C | ʵ������ȡ����O2���� | ������ˮ����������ƹ����У�����ˮ���ռ����������� |
| D | ��ȥ����ͭ��Һ�������������� | �������ͭ�ۣ���ַ�Ӧ����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
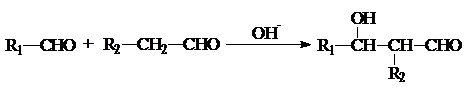 ���л���G��C20H18O4����һ�������ϳ�·�����£�
���л���G��C20H18O4����һ�������ϳ�·�����£�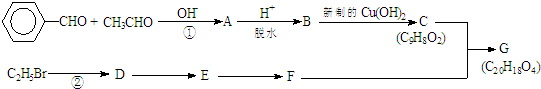
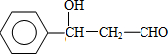 ��F �Ľṹ��ʽ��CH2OHCH2OH��
��F �Ľṹ��ʽ��CH2OHCH2OH�� +CH2OHCH2OH$��_{��}^{Ũ����}$
+CH2OHCH2OH$��_{��}^{Ũ����}$ +2H2O��
+2H2O��
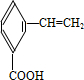 ��
�� ��
�� ��
��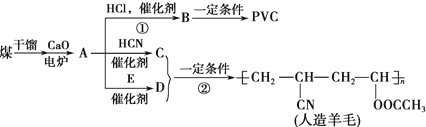
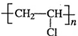 ��CH2C=HC-CN��
��CH2C=HC-CN��