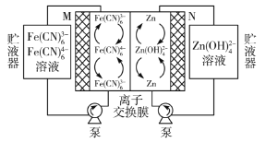
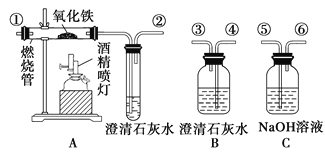
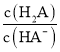题目内容
【题目】实验室用乙醇、浓磷酸和溴化钠反应来制备溴乙烷,其反应原理和实验的装置如下(反应需要加热,图中省去了加热装置),反应原理为:H3PO4(浓)+NaBr![]() +NaH2PO4+HBr,CH3CH2OH+HBr
+NaH2PO4+HBr,CH3CH2OH+HBr![]() CH3CH2Br+H2O, 有关数据见下表:
CH3CH2Br+H2O, 有关数据见下表:
物质名称 | 乙醇 | 溴乙烷 | 溴 |
状态 | 无色液体 | 无色液体 | 深红棕色液体 |
密度/gcm-3 | 0.79 | 1.44 | 3.1 |
沸点/℃ | 78.5 | 38.4 | 59 |
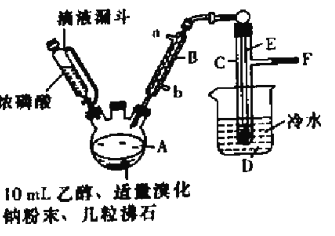
(1)A中加入沸石的作用是______________________.
(2)仪器B的名称为____________,该仪器的进水口为_______(填“a”或“b”)口.
(3)实验中用滴液漏斗代替分液漏斗的优点为_________________
(4)制取氢溴酸时,为什么不能用浓H2SO4代替浓磷酸?_________(用化学方程式表示).
(5)将C中的馏出液转入锥形瓶中,边振荡边逐滴滴入浓H2SO4以除去水等杂质,滴加浓硫酸约1~2mL,使溶液明显分层,再用分液漏斗分去硫酸层,将经硫酸处理后的溴乙烷转入蒸馏瓶,水浴加热蒸馏,收集到35~40℃馏分约10.0g,从乙醇的角度考虑,本实验所得溴乙烷的产率是______(保留三位有效数字)
【答案】防止液体瀑沸 球形冷凝管 b 平衡压强,使浓磷酸顺利流下 2HBr+H2SO4(浓)=Br2+SO2↑+2H2O 53.3%
【解析】
(1)液体加热时溶液发生暴沸;
(2)由仪器的结构可知B为球形冷凝管,为正确冷凝效果,应采取逆流原理通入冷凝水;
(3)滴液漏斗可以使漏斗的上方和下方的压强相等,起到平衡压强的作用;
(4)氢溴酸具有还原性,浓硫酸具有强氧化性,而浓磷酸没有氧化性;
(5)先依据乙醇的量计算溴乙烷的理论量,再依据溴乙烷的实际量计算产率。
(1)液体加热时溶液发生暴沸,加入沸石的目的是防止加热液体瀑沸,故答案为:防止液体瀑沸;
(2)由仪器的结构可知B为球形冷凝管,为正确冷凝效果,应采取逆流原理通入冷凝水,则B中进水口为b,故答案为:球形冷凝管;b;
(3)滴液漏斗可以使漏斗的上方和下方的压强相等,起到平衡压强的作用,便于浓磷酸顺利流下,而分液漏斗没有这个功能,故答案为:平衡压强,使浓磷酸顺利流下;
(4)氢溴酸具有还原性,浓硫酸具有强氧化性,能被浓硫酸氧化,生成溴单质,浓硫酸被还原成二氧化硫,反应的化学方程式为2HBr+H2SO4(浓)=Br2+SO2↑+2H2O,而浓磷酸没有氧化性,所以不能用浓H2SO4代替浓磷酸,故答案为:2HBr+H2SO4(浓)=Br2+SO2↑+2H2O;
(5)10mL乙醇的质量为0.79 gcm-3×10g=7.9g,物质的量为![]() =0.172mol,由化学方程式可知理论制得溴乙烷的物质的量为0.172mol,质量为0.172mol×109g/mol≈18.75g,则溴乙烷的产率为
=0.172mol,由化学方程式可知理论制得溴乙烷的物质的量为0.172mol,质量为0.172mol×109g/mol≈18.75g,则溴乙烷的产率为![]() ×100%=53.3%,故答案为:53.3%。
×100%=53.3%,故答案为:53.3%。