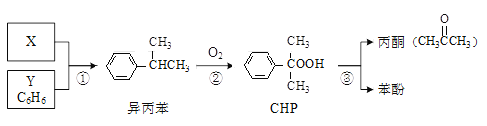
��Ŀ����
����Ŀ��ijѧϰС��Ϊ����֤SO2�Ļ�ԭ�Բ��ⶨ����SO2����������װ����ͼװ�á��ش��������⣺
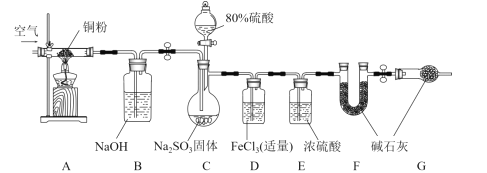
��1��C�з�����Ӧ�Ļ�ѧ����ʽ��________��Bװ�õ�������_________�����ڸ���װ��˵����ȷ����_________�����ţ���
a ��װ����������ҩƷ���������� b ʵ�鿪ʼ�����ʱ����Ҫͨ�����
c ����A��Bװ����Ϊ�˼�Сʵ����� d F��G�еļ�ʯ�ҿ��Ի�Ϊ��ˮCaCl2
��2����ͬѧ��Ϊ�ܿ���Կ���Dװ�õ���������������_________�������ӷ���ʽ��ʾ������ͬѧ��ʵ��ʱ���ֺͼ�ͬѧԤ�������һ�����ȳ����غ�ɫ������һ��ʱ����Ϊdz��ɫ�����Dz������Ϸ��ַ�Ӧ��Ϊ������
��һ����![]() ���췴Ӧ��
���췴Ӧ��
�ڶ�����![]() ������Ӧ��
������Ӧ��
�����Ҫ��֤��һ����Ӧ�ǿ췴Ӧ������������Dװ���еμ�����_________�Լ�����Ӧ��ʵ��������________��
��3��ѧϰС��ͨ��ʵ���D��Fװ���е�����������SO2��������D�м���������BaCl2��Һ��ַ�Ӧ������________��������ó���Ϊm1g��F��������Ϊm2g��C�в���SO2���������״���£�Ϊ_________L����ʵ�������У���Ȼ����ϵͳ���Ŀ���ԭ����______��
���𰸡�Na2SO3+H2SO4=Na2SO4+SO2��+H2O ϴ��ƿ����ƿ bc 2Fe3++SO2+2H2O=2Fe2++SO42-+4H+ ���軯�ػ�K3[Fe(CN)6] ��Һ�����غ�ɫʱû��������ɫ���� ���ˡ�ϴ�ӣ�������� ![]() C��Һ���ܽ��SO2δ������ų����������������
C��Һ���ܽ��SO2δ������ų����������������
��������
��ʵ����֤SO2�Ļ�ԭ�Բ��ⶨ����SO2��������Cװ���Ƿ���װ�ã��Ʊ�SO2��A��ͨ�������ʵ��֮ǰͨ�������ų�װ���еĿ�����ʵ��֮��ͨ�������ʹC�е�SO2����D��Fװ�ó�ַ�Ӧ����A��Bװ�����ų������е������������������ֹ�������飻Fװ��ͨ����ʯ������SO2���京����SO2���л�ԭ�ԣ����������������ԣ�����������ԭ��Ӧ����Һ���dz��ɫ����Ҫ��֤��һ��![]() �ǿ췴Ӧ�����Լ���Ƿ���������Fe2+����Һ���г�������ͨ�����ˡ�ϴ�ӡ���������õ�����������ͨ��SԪ���غ�����C�в�����SO2��������ݴ˷�����
�ǿ췴Ӧ�����Լ���Ƿ���������Fe2+����Һ���г�������ͨ�����ˡ�ϴ�ӡ���������õ�����������ͨ��SԪ���غ�����C�в�����SO2��������ݴ˷�����
(1)Cװ���Ƿ���װ�ã��Ʊ�SO2��������Ӧ�Ļ�ѧ����ʽ��Na2SO3+H2SO4=Na2SO4+SO2��+H2O��Bװ�������տ����е��������壬������ϴ��ƿ����ƿ����װ������������ҩƷ֮ǰ��������ԣ�a����ʵ�鿪ʼ�����ʱ����Ҫͨ�������ʵ��֮ǰͨ�������ų�װ���еĿ�����ʵ��֮��ͨ�������ʹC�е�SO2����D��Fװ�ó�ַ�Ӧ��b��ȷ��A��Bװ�����ų������е������������������ֹ����ʵ�飬����A��Bװ����Ϊ�˼�Сʵ����c��ȷ��F������SO2��SO2����ˮCaCl2����Ӧ��d����Ϊ��Na2SO3+H2SO4=Na2SO4+SO2��+H2O��ϴ��ƿ����ƿ��bc��
(2)Dװ�������������������ԣ�SO2���л�ԭ�ԣ�����������ԭ��Ӧ����Һ���dz��ɫ�����ӷ���ʽΪ2Fe3++SO2+2H2O=2Fe2++SO42-+4H+����Ҫ��֤��һ��![]() �ǿ췴Ӧ�����Լ���Ƿ���������Fe2+����ʵ��ǰ�μӼ������軯�ػ�K3[Fe(CN)6]������Һ�����غ�ɫʱû��������ɫ��������˵������Ϊ��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+�����軯�ػ�K3[Fe(CN)6]����Һ�����غ�ɫʱû��������ɫ������
�ǿ췴Ӧ�����Լ���Ƿ���������Fe2+����ʵ��ǰ�μӼ������軯�ػ�K3[Fe(CN)6]������Һ�����غ�ɫʱû��������ɫ��������˵������Ϊ��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+�����軯�ػ�K3[Fe(CN)6]����Һ�����غ�ɫʱû��������ɫ������
(3)D�м���������BaCl2��Һ��ַ�Ӧ������BaSO4�������������ˡ�ϴ�ӡ����������������ó���Ϊm1g��F��������Ϊm2g��˵��ʣ���SO2������Ϊm2g������ǰ��Sԭ���غ㣬n(SO2)=n(BaSO4)+n(ʣ��SO2)=![]() ����״���µ����Ϊ
����״���µ����Ϊ![]() ����ʵ�������У���Ȼ����ϵͳ���Ŀ���ԭ����C��Һ���ܽ��SO2δ������ų���������������ף���Ϊ�����ˡ�ϴ�ӡ����������
����ʵ�������У���Ȼ����ϵͳ���Ŀ���ԭ����C��Һ���ܽ��SO2δ������ų���������������ף���Ϊ�����ˡ�ϴ�ӡ����������![]() ��C��Һ���ܽ��SO2δ������ų���������������ף�
��C��Һ���ܽ��SO2δ������ų���������������ף�

����Ŀ��ʵ��С���Ʊ�������أ�K2FeO4����̽�������ʡ�
���ϣ�K2FeO4Ϊ��ɫ���壬����KOH��Һ������ǿ�����ԣ������Ի�������Һ�п��ٲ���O2���ڼ�����Һ�н��ȶ���
��1���Ʊ�K2FeO4���г�װ���ԣ�
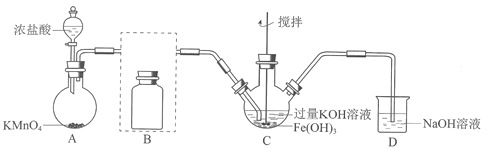
��AΪ��������װ�á�A�з�Ӧ����ʽ��________________���̱���ԭΪMn2+����
�ڽ�����װ��B�������������������Լ���_______
��C�еõ���ɫ�������Һ��C��Cl2�����ķ�Ӧ��
3Cl2+2Fe(OH)3+10KOH![]() 2K2FeO4+6KCl+8H2O�������________________��
2K2FeO4+6KCl+8H2O�������________________��
��2��̽��K2FeO4������
��ȡC����ɫ��Һ������ϡ���ᣬ��������ɫ���壬����Һa�������������к���Cl2��Ϊ֤���Ƿ�K2FeO4������Cl��������Cl2��������·�����
������ | ȡ����a���μ�KSCN��Һ����������Һ�ʺ�ɫ�� |
������ | ��KOH��Һ���ϴ��C�����ù��壬����KOH��Һ��K2FeO4�ܳ����õ���ɫ��Һb��ȡ����b���μ����ᣬ��Cl2������ |
i���ɷ���������Һ����֪a�к���______���ӣ��������ӵIJ��������ж�һ����K2FeO4��Cl����������������________________�������÷���ʽ��ʾ����
ii���������֤��K2FeO4������Cl������KOH��Һϴ�ӵ�Ŀ����________________��
�ڸ���K2FeO4���Ʊ�ʵ��ó���������Cl2________![]() �����������������������ʵ�������Cl2��
�����������������������ʵ�������Cl2��![]() ��������ǿ����ϵ�෴��ԭ����________________��
��������ǿ����ϵ�෴��ԭ����________________��
�����ϱ�����������Һ�е�������![]() ��
��![]() ����֤ʵ�����£�����Һb����MnSO4������H2SO4�Ļ����Һ�У�����Һ��dz��ɫ���������ܷ�֤��������
����֤ʵ�����£�����Һb����MnSO4������H2SO4�Ļ����Һ�У�����Һ��dz��ɫ���������ܷ�֤��������![]() ��
��![]() �����ܣ���˵�����ɣ������ܣ���һ�����ʵ�鷽����
�����ܣ���˵�����ɣ������ܣ���һ�����ʵ�鷽����
���ɻ���________________��