��Ŀ����
����Ԫ�ص���������(Se)����(Te)��Ԫ���ڻ������г����ֳ����ֻ��ϼۣ�������Ԫ�صĻ��������о�����������������Ҫ��;����ش��������⣺
(1) S���ʵij�����ʽΪS8���价״�ṹ����ͼ��ʾ��Sԭ�Ӳ��õĹ���ӻ���ʽ�� ��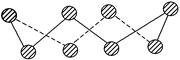
��2��Seԭ����Ԫ�����ڱ���λ��Ϊ ��
���̬ԭ�Ӻ���۵��ӵĹ����ʾʽΪ ��
��3����̬SeO3���ӵ����幹��Ϊ ��
��4��H+����H2O�γ�H3O+��H3O+�д��ڵĹ��ۼ�����Ϊ�� ��H3O+��H��O��H���DZ�H2O��H��O��H���Ǵ�ԭ��Ϊ_____________________________________________________
��1��sp3��2�֣� ��2���������ڵڢ�A�壻 ����2�֣���4�֣�
����2�֣���4�֣�
��3����ƽ�棩�����Σ�2�֣� ��4�����ԣ����ۣ�������λ������1�֣���2�֣���
H2O��Oԭ����2�Թ¶Ե��ӣ�H2O��ֻ��1�Թ¶Ե��ӣ��ų�����С��2�֣�
���������������1����S8���ӽṹ��֪����S8������Sԭ�ӳɼ����Ӷ���Ϊ2���µ��Ӷ���Ϊ2�����۲���Ӷ���Ϊ4�����Sԭ�Ӳ��õ��ӻ������ʽΪsp3��
��2��Seλ��Ԫ�����ڱ��������ڵڢ�A�壬ԭ������Ϊ34�����ݹ���ԭ����֪���������Ų�ʽΪ1s22s22p63s23p63d104s24p4�����Ը����������ԭ�������ع��������������ԭ����֪����̬ԭ�Ӻ���۵��ӵĹ����ʾʽΪ ��
��
��3�����ݼ۲���ӶԻ������ۿ�֪��SeO3����������ԭ�Ӻ��еŶԵ��Ӷ�������6��3��2����2��0������̬SeO3������Se�γ�3���ļ���û�йµ��Ӷԣ����Ը÷����γɵĿռ乹����ƽ�������Ρ�
��4��H+����H2O�γ�H3O+��H3O+�д��ڵĹ��ۼ�����Ϊ���ԣ����ۣ�������λ��������H2O��Oԭ����2�Թ¶Ե��ӣ�H2O��ֻ��1�Թ¶Ե��ӣ��ų�����С�����H3O+��H��O��H���DZ�H2O��H��O��H���Ǵ�
���㣺�����ӻ�������͡���������Ų����������ʽ�����ӿռ乹���Լ���ѧ�����жϵ�

�±���Ԫ�����ڱ���һ���֡��������е���ĸ�ֱ����һ�ֻ�ѧԪ�ء�
| a | | | |||||||||||||||
| b | | | | c | d | e | f | | |||||||||
| g | h | i | j | | k | l | m | ||||||||||
| n | | | | | | | o | | | | | | | | | | |
�Իش��������⣺
��1����д����Ԫ��o�Ļ�̬ԭ�ӵ����Ų�ʽ ��
��2��k�ڿ�����ȼ�ղ���ķ��ӹ���Ϊ ������ԭ�ӵ��ӻ���ʽΪ ���÷����� ������ԡ��Ǽ��ԡ������ӡ�
��3����10���ӵ�d���⻯����ӵ�VSEPRģ��Ϊ ��Ԫ��c��a��e��ԭ�Ӹ���1��2��1��������г�������ζ���ӣ��÷������� ������̼ԭ�ӡ�
��4��g��h��i����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ ����Ԫ�ط��ţ���
��5������Ԫ���е縺�������� ����ͼ�������ʾ��Ԫ�ط��ţ���Ԫ��k��������������Ƕ�Ӧ��ˮ���������ǿ��˳��Ϊ ��(�ѧʽ)
���ֶ�����Ԫ�ص����ʻ�ṹ��Ϣ���±����������Ϣ�ش��������⡣
| Ԫ�� | T | X | Y | Z |
| ���� �ṹ ��Ϣ | �����ں�������Ԫ�أ����䵥���dz�������ȼ���� | ����Ϊ˫ԭ�ӷ��ӣ������к���3�Թ��õ��Ӷԣ������µ������������ȶ�������ԭ�ӽϻ��á� | ��������������ɫ���塢������ǿ�� �����ڿ�����ȼ�շ�����ɫ�Ļ��档 | ��������Ԫ�صļ������а뾶��С |
��2��д��Zԭ�ӵĺ�������Ų�ʽ ��
��3��Z������������Ӧˮ����ĵ��뷽��ʽ ��
��4��Ԫ��T���Ԫ����ȣ��ǽ����Խ�ǿ���� ����Ԫ�ط��ű�ʾ�������б�������֤����һ��ʵ����
a�������·�������ɫ��T���ʵ���ɫ��
b��������T���⻯����ҷ�Ӧ������T�ĵ���
c������T�γɵĻ�������TԪ�س�����̬
d���Ƚ���Ԫ�صĵ�������������ʱ�õ��ӵ���Ŀ
��X��Y��Z��W���ֺ�14�����ӵ����ӣ���ṹ�ص����£�
| ���Ӵ��� | X | Y | Z | W |
| ԭ�Ӻ��� | ���� | ��ͬԪ�ع��ɵ����� | ͬԪ�ع��ɵ����� | ͬԪ�ع��ɵ����� |
| ���ӵĵ���� | 0 | 0 | ��������� | 0 |
(1)Aԭ�Ӻ����Xԭ�Ӷ�3�����ӣ�A��ԭ�ӽṹʾ��ͼ�� ����lmolX�������ᄃ���к��й��ۼ���ĿΪ ��
(2)Z���������ɵĻ�����ĵ���ʽΪ ��
(3)14gY��ȫȼ�շų���������141.5kJ��д��Yȼ�յ��Ȼ�ѧ����ʽ ��
(4)���W��Ԫ������������Ӧ��ˮ���������ͼ��ʾת����ϵ����Ϊ�������ʣ���Ӧ�����������������ԣ�

��д����Ԫ�������ڱ��е�λ�� ��
��д�����ڸ�������ˮ��Ӧ�Ļ�ѧ����ʽ ��
�����W��Ԫ�صļ��⻯�K������ˮ����Ҫԭ���� �����⻯����������Թ���һ��ȼ�ϵ�أ��������Һ��KOH���为���ĵ缫��ӦʽΪ ��
���в��ֶ���������Ԫ�ص��й���Ϣ�����±���
| Ԫ�ر�� | T | X | Y | Z | W |
| Ԫ�ص����ʻ�ԭ�ӽṹ��� | ����������Ԫ����ԭ�Ӱ뾶��� | ������ϵĵ������ȴ������1���ҵ��������� | �����13���˶�״̬��ͬ�ĵ��� | ����������Һ̬����������е�ϵͿ��Ȼ�����ĵ��� | ������5�ֲ�ͬ�����ĵ���������������δ�ɶԵĵ��� |
��1��Y�����������Ų�ʽ��__ __�����ĵ�����̼���ɵĻ�������ˮ��Ӧ���ɼ���Ͱ�ɫ������д���÷�Ӧ�Ļ�ѧ����ʽ__ ��
��2��Ԫ��T�ĵ�����ˮ��Ӧ�����ӷ���ʽ�� ��
�ڶ���������Ԫ���У�XԪ����������Ԫ�ص�ԭ�Ӱ뾶��С�����˳����_
��дԪ�ط��ţ���
��3��W�γɵ�һ�ֵ��ʣ���ʽ��Ϊ256��������CS2���õ��ʵĻ�ѧʽΪ___ __��������_ ___���壨д�������ͣ���
��4����ͼΪZԪ������������̬�⻯��R-H���ļ��ܴ�С���������Ԫ����̬�⻯����ܴ�С������Ĺ�ϵΪ_____ �������ּ�������
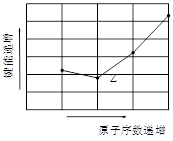
�±�������Ԫ�����ڱ����ֶ����ڵ�����Ԫ��
| | W | X | Y | |
| R | | | | Z |
��֪RΪ�ؿ��к������Ľ���Ԫ�ء�
��1��д��Z��ԭ�ӽṹʾ��ͼ________��
��2��W����ԭ���γ�6ԭ�ӷ��ӵĽṹ��ʽ_______��
��3����ϸRX��ĩ��Ӧ���ڴ��ģ���ɵ�·����������ԭ��ΪR2Y3��X2��W�ڸ����·�Ӧ�������ֻ���������ֻ������������Ԫ����ɣ���ԭ�Ӹ����Ⱦ�Ϊ1��1���䷴Ӧ�Ļ�ѧ����ʽΪ_______��
��4��X����������Ӧˮ������X��̬�⻯�ﷴӦ������������ˮ�У�������Һ����Ũ�ȴӴ�С��˳����_______��
��5�������������ȼ����(N2H4)��������N2O4��Ӧ����N2��ˮ������
��֪��N2(g)+2O2(g)=N2O4(l) ��H1=��195kJ?mol��1
��N2H4(l) +O2(g)=N2(g)+2H2O(g) ��H2=��534.2kJ?mol��1
д���º�N2O4��Ӧ���Ȼ�ѧ����ʽ______________��
��6���¶�ΪTʱ����2.0L�����ܱ������г���1.00 mol PCl5����ӦPCl5(g)
 PCl3(g)+Cl2(g)������һ��ʱ�䣨t)��ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
PCl3(g)+Cl2(g)������һ��ʱ�䣨t)��ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���| t/s | 0 | 50 | 150 | 250 | 350 |
| n(PCl3)/mol | 0 | 0.16 | 0.19 | 0.20 | 0.20 |
��ͬ�¶��£���ʼʱ�������г���1.00 mol PC15��0.20 mol PCl3��0.40 mol Cl2����Ӧ�ﵽƽ��ǰv(��) _______v(�棩���>����=����<������ԭ����_______��
��ͬԪ�ص�ԭ���ڷ������������ӵ�������С����һ����ֵx����ʾ�� xԽ����ԭ���������ӵ�����Խǿ�� ������ijЩ������Ԫ�ص�xֵ��
| Ԫ�ط��� | Li | Be | B | C | O | F | Na | Al | Si | P | S | Cl |
| xֵ | 0.98 | 1.57 | 2.04 | 2.55 | 3.44 | 3.98 | 0.93 | 1.61 | 1.90 | 2.19 | 2.58 | 3.16 |
��1��ͨ������xֵ�仯���ɣ�ȷ��Mg��xֵ��Χ��_______ < x(Mg) <_________��
��2���Ʋ�xֵ��ԭ�Ӱ뾶�Ĺ�ϵ��________________________________�����ݶ�����Ԫ�ص�xֵ�仯�ص㣬������Ԫ�����ʵ�_________________�仯���ɡ�
��3���ֱ�ָ���������ֻ���������Ԫ�صĻ��ϼۣ�HClO_________��HFO________��
��4��������ɸ������ǣ����ɼ�����ԭ����ӦԪ��x��ֵ�IJ�ֵ����x��������x>1.7ʱ��һ��Ϊ���Ӽ�������x<1.7ʱ��һ��Ϊ���ۼ������ƶ�AlBr3�л�ѧ��������________________��
��5��Ԥ��Ԫ�����ڱ��У�xֵ��С��Ԫ��λ��______����________��(������Ԫ�س���)��
(12��)�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����ش��������⣺
| �� ���� | IA | | 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | | | �� | �� | �� |
Ԫ���� ��������Ԫ�ط��ţ�
��2��Ԫ�آ���Ԫ�آ���ȣ���ķǽ����Խ�ǿ�����б�������֤����һ��ʵ���� ����
a�������¢ߵĵ��ʺ͢�ĵ���״̬��ͬ����
b������⻯��Ȣߵ��⻯���ȶ�
c��һ�������¢ߺ͢�ĵ��ʶ���������������Һ��Ӧ
d���ߵ��������ˮ����Ȣ���������ˮ����������
���һ����ʵ�飬֤��Ԫ�آ���Ԫ�آ�ķǽ�����ǿ����ֻд����Ӧ�Ļ�ѧ����ʽ����дʵ�鲽�裩______________________________________
��3���������Ԫ������������ˮ�������Ӧ�����ӷ���ʽΪ _____________________________________________________
��4���۵��⻯��ĵ���ʽΪ__________________
��5���٢ܢ�����Ԫ���γɵ����һ�ֻ����ﺬ�еĻ�ѧ��������