��Ŀ����
����Ŀ������ֲ���纣���������к��д����ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ������Ӻ�������ȡ�����������ͼ��
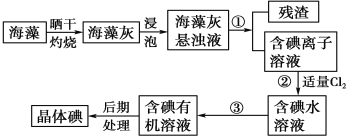
��1��ʵ���ұ��պ�������Ҫ���������е�________������ţ���
a �Թܡ�b �ձ���c ������d �����ǡ�e �����żܡ�f �ƾ���
��2��ָ����ȡ��Ĺ������йص�ʵ��������ƣ���__________����________��
��3����ȡ��Ĺ����У���ѡ����л��Լ�����_______��
A �ױ����ƾ���������������B ���Ȼ�̼����
C ���͡����� D ���͡�����
��4��Ϊʹ������е�����ת��Ϊ����л���Һ��ʵ�������ձ���������������ƿ���ƾ��ơ����ܡ�Բ����ƿ��ʯ�����Լ���Ҫ�ļг���������Ʒ����ȱ�ٵIJ���������__________��__________��
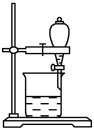
��5��С����CCl4��ȡ��ˮ�еĵ⣬����ͼ�ķ�Һ©���У��²�Һ���__________ɫ��
��6���Ӻ�����л���Һ����ȡ��ͻ����л��ܼ������뾭������ָ������ʵ��װ��ͼ�еĴ���֮����
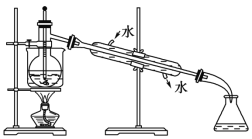
��____________________��
��_______________________________��
��____________________________��
���𰸡�cdef ���� ��ȡ����Һ B ��Һ©�� ��ͨ©�� �Ϻ� ȱʯ���� �¶ȼƲ嵽��Һ���� �����ܽ���ˮ�ķ���ߵ�
��������
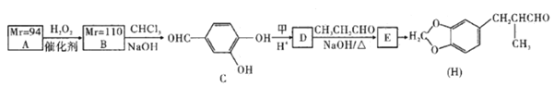
��ʵ��Ӻ������Ʊ��ⵥ�ʣ��Ƚ�����ɹ�ɡ����ճɺ���ң�Ȼ����ݳ����е����ӵ���Һ�����õ����Ӿ��н�ǿ�Ļ�ԭ�ԣ��������������������������������ɵⵥ�ʣ�Ȼ�����л��ܼ���ȡ����Һ�������Ƶõⵥ�ʡ�
��1�����պ�����Ҫ�������У��ƾ��ơ����żܡ������Ǽ���������ѡcdef��
��2�����������Թ������Һ���ù��˷������ӵ�ˮ�з���������л���Һ���÷�Һ�������������IJ��������ǹ��ˣ����IJ�����������ȡ����Һ���ʴ�Ϊ�����ˣ���ȡ����Һ��
��3��A���ƾ���ˮ���ܣ����Բ�������ȡ������A����
B�����Ȼ�̼����������ȡ������������������ȡ������B��ȷ��
C�������ˮ���ܣ��������������ȡ������C����
D�����ͺ�ˮ���ܣ����Ը��Ͳ�������ȡ������D����
�ʴ�Ϊ��B��
��4�����IJ����ǹ��˻���Ҫ��ͨ©�������IJ�������ȡ����Һ����Ҫ��Һ©�����ʴ�Ϊ����ͨ©������Һ©����
��5�����Ȼ�̼���ܶȴ���ˮ�Һ�ˮ�����ܣ����Ȼ�̼����ȡ�⣬�����л������·���ˮ���Ϸ���������Ȼ�̼��Һ���Ϻ�ɫ���ʴ�Ϊ���Ϻ죻
��6���¶ȼƲ�������¶ȣ������¶ȼ�ˮ����Ӧ��λ��������ƿ֧�ܿڴ����������е�ˮӦ��ѭ���½��ϳ���ԭ������ƿ��Ҫ��ʯ������������ƿ���Ȳ����ȣ��ʴ�Ϊ��ȱʯ�������¶ȼƲ嵽��Һ���У������ܽ���ˮ�ķ���ߵ���

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�����Ŀ��������(SO2Cl2)�����Ȼ������Ȼǻ�������������ҩƷ��Ⱦ�ϡ�������Լ��ȡ��й����ʵIJ����������±���
���� | �۵�/�� | �е�/�� | �������� |
SO2Cl2 | -54.1 | 69.1 | ����ˮ�⣬������������ ���ֽ⣺SO2Cl2 |
H2SO4 | 10.4 | 338 | ��ˮ���Ҳ��ֽ� |
ʵ�����ø�������Ķ�������������ϳ������ȣ�װ����ͼ��ʾ���г�������ʡ�ԣ�����ش��й����⣺

��1������A��ȴˮ�Ľ�ˮ��Ϊ________������a������b������
��2������B��ʢ�ŵ�ҩƷ��________����Ŀ����________________________��
��3��ʵ��ʱ��װ�ö��з�����Ӧ�����ӷ���ʽΪ______________________________��������6.72L������������£���ת�Ƶ��ӵ����ʵ���Ϊ________��
��4��װ�ñ���ʢ�ŵ��Լ���________����ȱ��װ���ң���ʵ����ɵ�Ӱ����_______________��
��5������������Ҳ����Һ̬�Ȼ��ᣨClSO3H�����´��ֽ��ã��÷�Ӧ�Ļ�ѧ����ʽΪ��2ClSO3H��H2SO4 +SO2Cl2���ӷֽ�����з���������ȵIJ�����______________��