��Ŀ����
12����Ҫ��ش��������⣮��1����Է�������Ϊ72�ҷе���͵������Ľṹ��ʽCH3C��CH3��2CH3
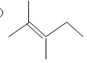
��2��2��3-����-2-��ϩ�ļ���ʽ
��3��
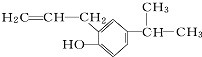 �ķ���ʽΪC12H16O��
�ķ���ʽΪC12H16O����4��
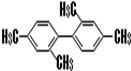 ������������11��̼ԭ�Ӵ���ͬһƽ���ϣ�
������������11��̼ԭ�Ӵ���ͬһƽ���ϣ���5���߾���
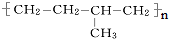 �ĵ���Ϊ
�ĵ���Ϊ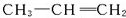 ��
�� ��
����6��A��B����Է�����������ȵ������������۶����Ժ��ֱ�����ϣ�ֻҪ���������������䣬��ȫȼ�պ���������CO2�������Ͳ��䣮��д��һ�����������������Ľṹ��ʽ��CH2=CH-CH3��CH2=CH2��A��BӦ�����������ʵ��ʽ��ͬ��
��7�������Ҵ������ᡢ����������������������ɫ��Һ�����õ�һ���Լ������Ƶ�������ͭ����Һ��
���� ��1���������ķ���ʽΪCxH��2x+2����������Է�������Ϊ72���г�����ʽ���м���xֵ��֧��Խ�࣬�е�Խ�ͣ�
��2�����ݼ���ʽ���ص����ش�
��3������ʽ����ʾ������Ԫ����������Լ�ԭ�Ӹ�����ʽ�ӣ�
��4���뱽��ֱ��������Cԭ�Ӵ��ڱ���Hԭ�ӵ�λ�ã�2������������Cԭ�Ӽ���Cԭ�Ӷ�λλ�õ�Cԭ�Ӵ���ͬһֱ�ߣ��ݴ��жϣ�
��5��������������ֻ���ĸ�̼ԭ�ӣ�������ԭ�ӣ���������˫���ĸ߾���䵥���Ϊ���֣������м仭�߶Ͽ���Ȼ���ĸ�����պϼ��ɣ�
��6�����������Ժ��ֱ�����ϣ�ֻҪ������������һ������ȫȼ������CO2������Ҳһ������˵�������ĸ������к�̼����ȣ�����ֻ����C��H���أ����Է����к�����Ҳ��ͬ���������ʽ��ͬ���ݴ˷������
��7������������ԣ������Ǻ���ȩ�����������Ʊ�������ͭ��Һ�ڼ��������·�Ӧ�������������ͭ���飮���ᡢ�Ҵ������������������Ƿֱ������Cu��OH�� 2����Һ��ϣ�����ֱ�Ϊ��ɫ��Һ������Ӧ���ֲ㡢����Ӧ�ֲ㡢ש��ɫ�������Դ������
��� �⣺��1���������ķ���ʽΪCxH��2x+2����
��14x+2=72�����x=5��
���Ը������ķ���ʽΪC5H12��
����ʽΪC5H12��ͬ���칹����������5��̼ԭ�ӵģ�CH3CH2CH2CH2CH3��
������4��̼ԭ�ӵģ�CH3CH��CH3��CH2CH3��
������3��̼ԭ�ӵģ�CH3C��CH3��2CH3��
֧��Խ�࣬�е�Խ�ͣ���CH3C��CH3��2CH3���ʴ�Ϊ��CH3C��CH3��2CH3��
��2��ֻ�ü�������ʾ̼�ܣ���������֮���һ��˫����һ������֮��ļн�Ϊ120?��һ��������һ������֮��ļн�Ϊ180?���������е�̼�����̼ԭ�Ӽ���̼ԭ����������ԭ�Ӿ�ʡ�ԣ���������ԭ�Ӽ�����ԭ����������ԭ���뱣���������ַ�ʽ��ʾ�ĽṹʽΪ����ʽ��2��3-����-2-��ϩ�ļ���ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��3������ʽ����ʾ������Ԫ����������Լ�ԭ�Ӹ�����ʽ�ӣ����л����й���12��̼ԭ�Ӻ�16��Hԭ���Լ�1����ԭ�ӣ��ʷ���ʽΪ��C12H16O���ʴ�Ϊ��C12H16O��
��4���뱽��ֱ��������Cԭ�Ӵ��ڱ���Hԭ�ӵ�λ�ã����ڱ����γɵ�ƽ�棬2������������Cԭ�Ӽ���Cԭ�Ӷ�λλ�õ�Cԭ�Ӵ���ͬһֱ�ߣ���������2+6+3=11��Cԭ�Ӵ���ͬһƽ�棬�ʴ�Ϊ��11��
��5��������������ֻ��̼ԭ�Ӳ�����̼̼˫���ṹ�ĸ߾��������ǡ���˫�����ĸ�̼����˫��������̼�����߶Ͽ���Ȼ����պϣ�������˫������������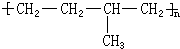 �ĵ���Ϊ��
�ĵ���Ϊ��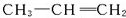 ��
�� ���ʴ�Ϊ��
���ʴ�Ϊ��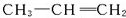 ��
�� ��
��
��6��ϩ����ͨʽΪCnH2n�������ʽΪCH2��������������ϩ����ϣ�ֻҪ������������һ������ȫȼ�պ���������CO2�������Ͳ��䣬�磺CH2=CH-CH3��CH2=CH2��
��Ȳ�ͱ������ʽ����CH�����Զ����ֻҪ������������һ������ȫȼ�պ���������CO2�������Ͳ��䣻�����������Ժ��ֱ�����ϣ�ֻҪ������������һ������ȫȼ������CO2������Ҳһ����˵����������ַ����к�̼����ȣ���������ֻ����C��HԪ�أ�������Ҳ��ȣ�������ʵ��ʽһ����ͬ��
�ʴ�Ϊ��CH2=CH-CH3��CH2=CH2��ʵ��ʽ��ͬ��
��7����������������ͭ���Ҵ������������ܽ�������ͭ������ʱ����������Һ������ש��ɫ�����������Ҵ������ᡢ���������������Ƿֱ������Cu��OH�� 2����Һ��ϣ�����ֱ�Ϊ����Ӧ���ֲ㡢��ɫ��Һ������Ӧ�ֲ㡢ש��ɫ����������ͬ���ɼ��𣬹ʴ�Ϊ�����Ƶ�������ͭ����Һ��
���� ���⿼���л���ȼ�յ��йؼ��㣬��Ŀ�Ѷ��еȣ�ע�����ճ����л���ȼ�յ�ͨʽ�����㷽������ȷ���������ϢΪ�����Ĺؼ����������������ѧ���ķ�����������������ѧ����������

 �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д� 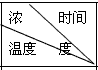 | 0 | 10 | 20 | 30 | 40 | 50 | 60 | |
| 1 | 800�� | 1.0 | 0.80 | 0.67 | 0.57 | 0.50 | 0.50 | 0.50 |
| 2 | 800�� | C1 | 0.60 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| 3 | 800�� | C2 | 0.92 | 0.75 | 0.63 | 0.60 | 0.60 | 0.60 |
| 4 | 800�� | 1.0 | 1.0 | 0.40 | 0.25 | 0.20 | 0.20 | 0.20 |
��1����ʵ��1����Ӧ��10��20����ʱ����ƽ������Ϊ0.013mol/��L•min����
��2����ʵ��2����Ӧ��20���Ӿʹﵽƽ�⣬���Ʋ�ʵ��2�л������������Ǵ�����
| A�� | 238U������92������ | B�� | 238U��ԭ������ԼΪ12C��238�� | ||
| C�� | 238Uԭ�Ӻ�����146������ | D�� | 238U��UԪ�ص�һ�ֺ��� |
| A�� | �÷�Ӧ���ʱ�Ϊ��ֵ | |
| B�� | ���º����£���������CO2�������H2��ת���� | |
| C�� | �����¶ȣ������淴Ӧ���ʾ����� | |
| D�� | �÷�Ӧ��ѧ����ʽΪ��CO+H2O$?_{��}^{����}$ CO2+H2 |
| A�� | W��Z��Y��X��ԭ�Ӱ뾶���μ�С | |
| B�� | Y�ֱ���Z��W�γɵĻ������л�ѧ��������ͬ | |
| C�� | X������������Ӧ��ˮ��������Ա�W���� | |
| D�� | Y����̬���⻯������ȶ��Ա�W��ǿ |
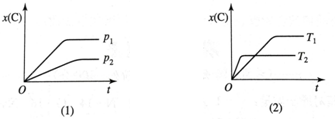
| A�� | ���ȷ�Ӧ��m+n��p | B�� | ���ȷ�Ӧ��m+n��p | C�� | ���ȷ�Ӧ��m+n��p | D�� | ���ȷ�Ӧ��m+n��p |

��I��A��B����������ˮ���������������ֱ�պA��B��Ũ��Һ����������ῴ�����̣�
��II����������һ�������·�Ӧ���й�����Ϊ��
| ��Ŀ | �� | �� | A |
| ��ʼʱ | 3mol/L | 3mol/L | 0 |
| 2sĩ | 1.8mol/L | 2.6mol/L | 0.8mol/L |
��1�����ĵ���ʽΪ��
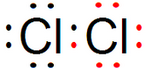
��2�������ҷ�Ӧ����A������Ϊ��v���ף�=0.6mol/��L•s����
��3��д��C��NaOH��Һ��Ӧ����A�����ӷ���ʽNH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$H2O+NH3����
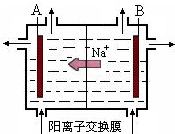 ���ϵ����нϷḻ��ʳ�ο�͵�����Դ���ʺϽ�����͵��ȼҵ����ͼ���ö��Ե缫��ⱥ��ʳ��ˮ��ԭ��ʾ��ͼ����ش��������⣺
���ϵ����нϷḻ��ʳ�ο�͵�����Դ���ʺϽ�����͵��ȼҵ����ͼ���ö��Ե缫��ⱥ��ʳ��ˮ��ԭ��ʾ��ͼ����ش��������⣺