��Ŀ����
6��A��B��C��D���ֶ���������Ԫ�ص�ԭ�������������ӣ���ԭ�Ӻ���L���Ӳ�ĵ������ֱ�Ϊ0��5��8��8�����ǵ�������������Ϊ18����������ش��������⣺
��1��д��DԪ�ص������ȼ������ڱ��е�λ�õ������ڵڢ�A��
��2��A��B��Ԫ�ؿ����γɻ�����B2A4��д���û�����ĵ���ʽ
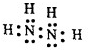
��3���Ƚ�B��C�ļ���̬�⻯����۷е�ߵͣ��ѧʽ��NH3��PH3��ԭ���ǰ�������֮�����������Ƚ������ӵĻ�ԭ��A-����D-������ڻ�С�ڣ�
��4��������CD5��������ˮ��Ӧ���Եõ������ᣬд���÷�Ӧ�Ļ�ѧ����ʽPCl5+4H2O=5HCl+H3PO4��
���� A��B��C��D��Ϊ�Ķ���������Ԫ�أ�ԭ�������������ӣ���ԭ�Ӻ���L���Ӳ�ĵ������ֱ�Ϊ0��5��8��8����A��HԪ�أ�B��NԪ�أ�C��D��������Ԫ�أ����ǵ�����������֮��Ϊ18��C��D����������֮����18-1-5=12������������ֻ��Ϊ5��7����Cԭ������С��D����C��PԪ�ء�D��ClԪ�أ��ݴ˽��
��� �⣺A��B��C��D��Ϊ�Ķ���������Ԫ�أ�ԭ�������������ӣ���ԭ�Ӻ���L���Ӳ�ĵ������ֱ�Ϊ0��5��8��8����A��HԪ�أ�B��NԪ�أ�C��D��������Ԫ�أ����ǵ�����������֮��Ϊ18��C��D����������֮����18-1-5=12������������ֻ��Ϊ5��7����Cԭ������С��D����C��PԪ�ء�D��ClԪ�أ�
��1��DԪ�ص����ƣ��ȣ������ڱ��е�λ�ã��������ڵڢ�A�壬�ʴ�Ϊ���ȣ��������ڵڢ�A�壻
��2��A��B��Ԫ�ؿ����γɻ�����ΪN2H4���û�����ĵ���ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3��B��C�ļ���̬�⻯��ֱ�ΪNH3��PH3�����ڰ�������֮�������������۷е㣺NH3��PH3���ǽ�����H��Cl�������ӵĻ�ԭ��H- ����Cl-��
�ʴ�Ϊ��NH3��PH3����������֮�������������ڣ�
��4��������PCl5��������ˮ��Ӧ���Եõ������ᣬӦ�������������ᣬ�÷�Ӧ�Ļ�ѧ����ʽΪ��PCl5+4H2O=5HCl+H3PO4��
�ʴ�Ϊ��PCl5+4H2O=5HCl+H3PO4��
���� ���⿼��ԭ�ӽṹ��Ԫ�����ʣ��漰����ʽ��Ԫ��������Ӧ�á�����ȣ���ȷ�ж�Ԫ���ǽⱾ��ؼ����ѶȲ���

 ������������ϵ�д�
������������ϵ�д�| A�� | ���ʵ�����֮��Ϊ9��1 | B�� | ԭ����֮��Ϊ1��1 | ||
| C�� | ������֮��Ϊ1��1 | D�� | ������֮��Ϊ5��1 |
| A�� | ��ʽ�ζ��ܵ����յ�ԣ����Ӷ��� | |
| B�� | ��ƿ������ˮϴ��δ���� | |
| C�� | ��ʽ�ζ���������ˮϴ��δ�ñ�Һ��ϴ | |
| D�� | ��ʽ�ζ��ܵζ����յ���ּ��촦�����ݣ�ԭ�������ݣ� |
| A�� | ��ϩ�ܷ����ӳɷ�Ӧ | |
| B�� | ��ϩ������ˮ��Ҳ�������л��ܼ� | |
| C�� | �������Ը��������Һ������ϩ����� | |
| D�� | ��ϩ�IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־ |
| A�� | CH4 | B�� | CH3Cl | C�� | C2H6 | D�� | C2H4 |
| A�� | c��H+��+c��NH4+ ��=c��OH- ��+c��HCO3-��+2c��CO32-�� | |
| B�� | c��Na+ ��=c��HCO3-��+c��CO32-��+c��H2CO3�� | |
| C�� | $\frac{{K}_{w}}{c��{H}^{+}��}$��1.0��10-7mol/L | |
| D�� | c��Cl-����c��NH4+����c��HCO3-����c��CO32-�� |
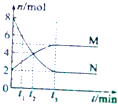 �о���ѧ��Ӧ�Ŀ������Ⱦ���ʮ����Ҫ�����壬��ش��������⣺
�о���ѧ��Ӧ�Ŀ������Ⱦ���ʮ����Ҫ�����壬��ش��������⣺ ��
��

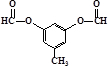
 ��д��F�����NaOH����ʱ��Ӧ�Ļ�ѧ����ʽ
��д��F�����NaOH����ʱ��Ӧ�Ļ�ѧ����ʽ ��
�� ��
��  ��
��