��Ŀ����
����Ŀ������ʱ����2 mol A��2 mol B����Ͷ��̶��ݻ�Ϊ2 L���ܱ������з�����Ӧ��2A(g)+B(g)![]() xC(g)+D(s)��10 sʱ�����A�����ʵ���Ϊ1.7 mol��0��10 s��C�ķ�Ӧ����Ϊ0.0225 mol��L��1��s��1��40 sʱ��Ӧǡ�ô���ƽ��״̬����ʱB��ת����Ϊ20%������д���пհף�
xC(g)+D(s)��10 sʱ�����A�����ʵ���Ϊ1.7 mol��0��10 s��C�ķ�Ӧ����Ϊ0.0225 mol��L��1��s��1��40 sʱ��Ӧǡ�ô���ƽ��״̬����ʱB��ת����Ϊ20%������д���пհף�
(1)x=________��
(2)ƽ��ʱ������B���������Ϊ________��
(3)���¶��´˷�Ӧ��ƽ�ⳣ������ʽΪ_____________����ֵ��__________��
(4)���и����ܱ�ʾ�÷�Ӧ�ﵽƽ��״̬����________________________��
A������A�����ʵ���������D�����ʵ���֮��Ϊ2��1
B��������A��B�����ʵ���n(A)��n(B) =2��1
C�������ƽ����Է����������ٱ仯
D��ѹǿ���ٱ仯
E�������ܶȲ��ٱ仯
���𰸡�3 40% 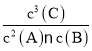 0.75 CE
0.75 CE
��������
(1)����A����ʼ�����ʵ�����10 sʱ�����ʵ�������A��Ӧ�����ʵ���������C���ʵ����ʼ�����n(C)���������ʵ����仯��֮�ȵ��ڻ�ѧ������֮�ȼ���x��ֵ��
(2)����B��ת���ʼ�����μӷ�Ӧ��B�����ʵ������������ʷ�Ӧת����ϵ����ƽ��ʱ����ɵ����ʵ�������������ƽ��ʱ�ܵ����ʵ���������������������ڼ���B�����������ע��D�ǹ��壻
(3)ƽ�ⳣ��ָ������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ��
�����ƽ��ʱ����ֵ�Ũ�ȣ�����ƽ�ⳣ������ʽ����ƽ�ⳣ����ע��D�ǹ��壻
(4)��ʾ�÷�Ӧ�ﵽƽ��״̬�����淴Ӧ������ͬ�����ɷֵĺ������ֲ��䡣
(1)A�����ʵ����仯����n(A)=2 mol-1.7 mol=0.3 mol����n(C)=0.0225 mol/(Ls)��10 s��2 L=0.45 mol���������ʵ����仯��֮�ȵ��ڻ�ѧ������֮�ȣ���0.3 mol��0.45 mol=2��x�����x=3��
(2)ƽ��ʱ�μӷ�Ӧ��B�����ʵ���Ϊ2 mol��20%=0.4 mol������ݷ�Ӧ����ʽ2A(g)+B(g)![]() 3C(g)+D(s)�����ʷ�Ӧת����ϵ����֪A��Ӧ�����ʵ���Ϊ0.8 mol����Ӧ����C�����ʵ���Ϊ1.2 mol����ƽ��ʱ������������ʵ���n(A)=2 mol-0.8 mol=1.2 mol��n(B)=2mol-0.4 mol=1.6 mol��n(C)=1.2 mol������ƽ��ʱB���������Ϊ
3C(g)+D(s)�����ʷ�Ӧת����ϵ����֪A��Ӧ�����ʵ���Ϊ0.8 mol����Ӧ����C�����ʵ���Ϊ1.2 mol����ƽ��ʱ������������ʵ���n(A)=2 mol-0.8 mol=1.2 mol��n(B)=2mol-0.4 mol=1.6 mol��n(C)=1.2 mol������ƽ��ʱB���������Ϊ![]() ��100%=40%��
��100%=40%��
(3)���淴Ӧ2A(g)+B(g)![]() 3C(g)+D(s)��ƽ�ⳣ��K=
3C(g)+D(s)��ƽ�ⳣ��K=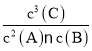 ����(2)�м����֪��ƽ��ʱA��B��C��Ũ�ȷֱ�Ϊc(A)=
����(2)�м����֪��ƽ��ʱA��B��C��Ũ�ȷֱ�Ϊc(A)=![]() =0.6 mol/L��c(B)=
=0.6 mol/L��c(B)=![]() =0.8 mol/L��c(C)=
=0.8 mol/L��c(C)=![]() =0.6 mol/L������ƽ�ⳣ��K=
=0.6 mol/L������ƽ�ⳣ��K=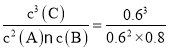 =0.75��
=0.75��
(4)A������A�����ʵ���������D�����ʵ���֮��Ϊ2��1��ֻ�ܱ�ʾ��Ӧ������У����ܾݴ��жϷ�Ӧ�Ƿ���ƽ��״̬��A���������⣻
B��������A��B�����ʵ��� n(A)��n(B)=2��1�����ܱ�ʾ�����淴Ӧ������ͬ����Ӧ���ܴ���ƽ��״̬��Ҳ����δ����ƽ��״̬��B���������⣻
C����Ӧǰ�����������仯������������ʵ������䣬�����ƽ����Է����������ٱ仯��˵����Ӧ�����淴Ӧ������ͬ����Ӧ�ﵽƽ��״̬��C�������⣻
D����Ӧʱ�����������ķ�Ӧ��ѹǿ���ٱ仯������ô��Ӧ�ﵽƽ�⣬D���������⣻
E���ܶȵ��������������������������Ӧǰ������������䣬��Ӧ�����ɵ�D�ǹ��壬�����ܶȲ��ٱ仯˵����Ӧ�ﵽƽ�⣬E�������⣻
�ʺ���ѡ����CE��

����Ŀ��CO��H2����Ϊ��Դ�ͻ���ԭ�ϣ�Ӧ��ʮ�ֹ㷺��������ú�������������ɵ�CO��H2���Ʊ��״���
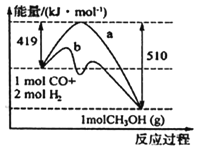
(1)�����ͼʾд���÷�Ӧ���Ȼ�ѧ����ʽ��_______________��ͼ��ʹ�ô���������_______(����a������b������
(2)��֪��C(s)+O2(g)=CO2(g) ��H1=��393.5 kJ/mol
C(s)+H2O(g)=CO(g)+H2(g) ��H2=+131.3 kJ/mol
��ӦCO(g)+H2(g)+O2(g)=H2O(g)+CO2(g)����H=______________kJ/mol��
(3)��ӦCO(g)+H2O(g)![]() H2(g)+CO2(g)��ƽ�ⳣ�����¶ȵı仯���±���ʾ��
H2(g)+CO2(g)��ƽ�ⳣ�����¶ȵı仯���±���ʾ��
�¶�/�� | 400 | 500 | 830 | 1000 |
ƽ�ⳣ��K | 10 | 9 | 1 | 0.6 |
�ٴ��ϱ������ƶϣ��˷�Ӧ��__________(����������������)�ȷ�Ӧ��
����830 ���£�����ʼʱ������ܱ������г���CO��H2O��Ϊ1 mol����ﵽƽ���CO��ת����Ϊ___________________��
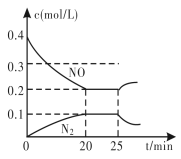
(4)�����������ϵĴ�ת�����������·�Ӧ��2NO(g)+2CO(g)![]() N2(g)+2CO2(g) ��H<0����һ���¶��£���һ������NO��CO����2 L�̶��ݻ��������У��ش��������⣺
N2(g)+2CO2(g) ��H<0����һ���¶��£���һ������NO��CO����2 L�̶��ݻ��������У��ش��������⣺
����˵���÷�Ӧ�ﵽƽ��״̬����_____________(����ĸ���)��
A. 2v��(NO)=v��(N2) B. ��������ƽ����Է����������ֲ���
C. ������ѹǿ���ٱ仯 D. ��H���ֲ���
E.���������ܶȲ��ٱ仯
�ڴӷ�Ӧ��ʼ��5 min��������0.08 mol N2����5 min��v(CO)=__________mol/(L��min)��
��25 minʱ������Ũ�ȱ仯��ͼ��ʾ����ı������������________(����ĸ���)��
A. ��С������� B. ����NO��Ũ��
C. �����¶� D. �����¶�
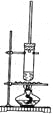
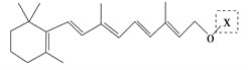
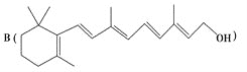
����Ŀ������ȩ��ҽҩ��Ⱦ�ϡ����ϵ���ҵ���Ź㷺��Ӧ�á�ʵ����ͨ����ͼ��ʾ�������ɼױ������Ʊ�����ȩ��
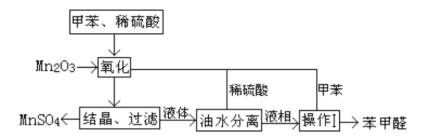
�Իش��������⣺
��1��Mn2O3�����ױ��ķ�Ӧ��Ҫ���Ͻ��裬�����������___��
��2���ױ���������õ��Ļ����ͨ���ᾧ�����˽��з��룬�ù������轫�������ȴ����Ŀ��___��
��3��ʵ������У���ѭ��ʹ�õ����ʷֱ�Ϊ___��___��
��4��ʵ���з���ױ��ͱ���ȩ���õIJ���I��___________��
��5��ʵ���з��֣���Ӧʱ�䲻ͬ����ȩ�IJ���Ҳ��ͬ(���ݼ��±�)��
��Ӧʱ��/h | 1 | 2 | 3 | 4 | 5 |
����ȩ����/% | 76.0 | 87.5 | 83.6 | 72.5 | 64.8 |
���ϱ���ȩ�Ľṹ����������Ӧʱ�����ʱ������ȩ�����½���ԭ��___��