��Ŀ����
3���±������ڱ��е�һ���֣�����A-I�����ڱ��е�λ�ã��ڣ�1������4��С����Ԫ�ط��Ż�ѧʽ�ش𣬣�5������8��С�ⰴ��ĿҪ��ش�| �� ���� | I A | ��A | ��A | ��A | ��A | ��A | ��A | O |
| 1 | A | |||||||
| 2 | D | E | G | H | J | |||
| 3 | B | C | F | I |
��2������������ˮ���������ǿ����NaOH��������ǿ����HClO4�������Ե���Al��OH��3��
��3��A�ֱ���D��E��F��H��I�γɵĻ������У����ȶ���HF��
��4����B��C��E��F��H��I�У�ԭ�Ӱ뾶������Na��
��5��D��G��ɻ�����ĵ���ʽ
 ��
����6��A��E��ɻ�����ɷ���Ũ������ﲻ�ܣ�ԭ���ǣ�2NH3+H2SO4=��NH4��2SO4���û�ѧ����ʽ�ش�
��7��B��G�γ�1��1�ͻ������к��еĻ�ѧ���������Ӽ������ۼ���
��8��B������������ˮ�����C��������������Ӧ�����ӷ���ʽAl��OH��3+OH-=AlO2-+2H2O��
���� ����Ԫ�������ڱ��е�λ��֪��A-J�ֱ���H��Na��Al��C��N��P��O��F��Ne��ClԪ�أ�
��1��ϡ����������ã��仯ѧ�������ȶ���O��FԪ��ֻ�и����ϼ�û�������ϼۣ���Ԫ�����ڱ��У��ǽ�������ǿ��Ԫ��λ�����ڱ����Ͻǣ�ϡ��������⣩����������ǿ��Ԫ��λ�����ڱ����½ǣ�
��2��Ԫ�صĽ�����Խǿ��������������ˮ�������Խǿ���ǽ�����Խǿ��������������ˮ��������Խǿ��O��FԪ�س��⣩�������Ե�������������
��3��Ԫ�صķǽ�����Խǿ�����⻯��Խ�ȶ���
��4��ͬһ����Ԫ�أ�ԭ�Ӱ뾶����ԭ�������������С��ԭ�ӵ��Ӳ���Խ����ԭ�Ӱ뾶Խ��
��5��D��G�γɵĻ�������CO2��Cԭ�Ӻ�ÿ��Oԭ���γ����Թ��õ��Ӷԣ�
��6��A��E�γɵ��⻯���ǰ��������ڼ������壬�ܺ��ᷴӦ��
��7��B��G�γ�1��1�ͻ�����ΪNa2O2���������������Ӻ���������֮��������Ӽ���O-Oԭ��֮����ڹ��ۼ���
��8��B������������ˮ������NaOH��C�������������Al��OH��3�������������������������������ǿ�ǿ����Һ��
��� �⣺����Ԫ�������ڱ��е�λ��֪��A-J�ֱ���H��Na��Al��C��N��P��O��F��Ne��ClԪ�أ�
��1��ϡ����������ã��仯ѧ�������ȶ���O��FԪ��ֻ�и����ϼ�û�������ϼۣ���Ԫ�����ڱ��У��ǽ�������ǿ��Ԫ��λ�����ڱ����Ͻǣ�ϡ��������⣩����������ǿ��Ԫ��λ�����ڱ����½ǣ������⼸��Ԫ���У���ѧ��������õ���Ne��ֻ�и���û�������ϼ۵���O��FԪ�أ���������ǿ�ĵ�����F2����ԭ����ǿ�ĵ�����Na��
�ʴ�Ϊ��Ne��O��F��F2��Na��
��2��Ԫ�صĽ�����Խǿ��������������ˮ�������Խǿ����������ǿ����Na�����Լ�����ǿ����NaOH��
�ǽ�����Խǿ��������������ˮ��������Խǿ��O��FԪ�س��⣩������������ˮ����������ǿ����HClO4�������Ե���Al��OH��3��
�ʴ�Ϊ��NaOH��HClO4��Al��OH��3��
��3��Ԫ�صķǽ�����Խǿ�����⻯��Խ�ȶ����ǽ�������ǿ����FԪ�أ���HF���ȶ�����ǿ���ʴ�Ϊ��HF��
��4��ͬһ����Ԫ�أ�ԭ�Ӱ뾶����ԭ�������������С��ԭ�ӵ��Ӳ���Խ����ԭ�Ӱ뾶Խ������ԭ�Ӱ뾶������Na���ʴ�Ϊ��Na��
��5��D��G�γɵĻ�������CO2��Cԭ�Ӻ�ÿ��Oԭ���γ����Թ��õ��Ӷԣ������ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��6��A��E�γɵ��⻯���ǰ��������ڼ������壬�ܺ����ᷴӦ��������泥����Բ�����Ũ��������Ӧ����ʽΪ2NH3+H2SO4=��NH4��2SO4��
�ʴ�Ϊ�����ܣ�2NH3+H2SO4=��NH4��2SO4��
��7��B��G�γ�1��1�ͻ�����ΪNa2O2���������������Ӻ���������֮��������Ӽ���O-Oԭ��֮����ڹ��ۼ������Ժ������Ӽ����ۼ����ʴ�Ϊ�����Ӽ������ۼ���
��8��B������������ˮ������NaOH��C�������������Al��OH��3�������������������������������ǿ�ǿ����Һ�������������������Ʒ�Ӧ����ƫ�����ƺ�ˮ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɣ�Ϊ�߿���Ƶ�㣬��ȷԪ�����ڱ��ṹ�����ʽṹ��Ԫ�������ɡ��������ʵȼ��ɽ��ע���������������ԣ���Ŀ�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ����֮���м�� | B�� | �����ڲ����˶� | ||
| C�� | ���ӵ��������������С | D�� | ������ԭ�ӹ��� |
| A�� | SO${\;}_{3}^{2-}$ | B�� | CH3COO- | C�� | Fe2+ | D�� | Al3+ |
| A�� | 431kJ•mol-1 | B�� | 945.6kJ•mol-1 | C�� | 649kJ•mol-1 | D�� | 896kJ•mol-1 |
| A�� | 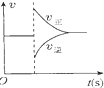 ͼ�ɱ�ʾ��ƽ��N2+3H2?2NH3��ѹ��ͬʱ�Ƴ�����NH3ʱ�����ʱ仯 | |
| B�� | 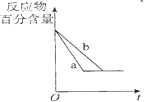 ͼ��a��b����ֻ�ɱ�ʾ��ӦH2��g��ʮI2��g��?2HI��g�����д��������������½���ƽ��Ĺ��� | |
| C�� | 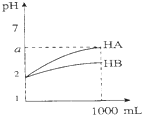 ͼ��ʾ�����½���1 ml pH=2��HA��HB��������Һ��ˮϡ�͵��������2��a��5�������ᶼΪ���� | |
| D�� | 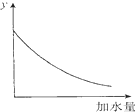 ͼ�е�y�ɱ�ʾ��O.1 mol/lϡ�����ˮϡ��ʱ��Һ���������ı仯��� |
| A�� | ��Ȼ������Ҫ�ɷ� | B�� | ʯ�;������ѻ��IJ��� | ||
| C�� | ���������䡱������ | D�� | ú���е���˹��ը |
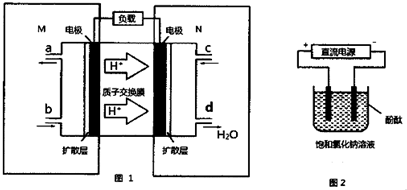
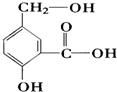

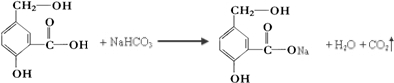
 ��
��