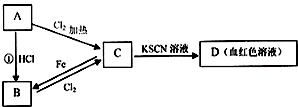
��Ŀ����
�Իش��������⣺
��ָ������ʵ����Ʒ������(��ϴ�Ӹɾ�)ʹ��ʱ�ĵ�һ��������
��ʯ����ֽ(������������)
������ƿ
 (2)����ʵ������ѡ�õ�����������Լ����ۺ�������____________��
(2)����ʵ������ѡ�õ�����������Լ����ۺ�������____________��
A. ��������ƽ����5.85 g�Ȼ��ƾ���
 B. ��������ˮ��pH��ֽ���Ϳ��Լ���pH��ȵ�H2SO4��Һ��CH3COOH��Һ
B. ��������ˮ��pH��ֽ���Ϳ��Լ���pH��ȵ�H2SO4��Һ��CH3COOH��Һ
C. �ü�ʽ�ζ�����ȡ25.00 mL���������Һ
 D. �����ô����������������ơ�̼����
D. �����ô����������������ơ�̼����
E. ��Ͳ��Һ���������Ϊ10.0 mLʱ��ȫ�������ձ��ڵ�ʵ�����Ҳ��10.0 mL
 F. 100 mL����ƿ��Һ�����ôﵽ�̶���ʱ��ȫ�������ձ��ڵ�ʵ�����Ҳ��Ϊ100 mL
F. 100 mL����ƿ��Һ�����ôﵽ�̶���ʱ��ȫ�������ձ��ڵ�ʵ�����Ҳ��Ϊ100 mL
(3)����ȡ15.00 mL Na2CO3��Һ��Ӧѡ�õ�������_________________��
 (4)������ͭ������ᾧˮ�����IJⶨʵ���У�������������Ҫ����___________�Ρ�
(4)������ͭ������ᾧˮ�����IJⶨʵ���У�������������Ҫ����___________�Ρ�
���𰸡�
�Ţٽ�ʯ����ֽ��ʪ �ڼ�������ƿ�Ƿ�©ˮ(2)B D E (3)��ʽ�ζ��� (4) 4
��������

��ϰ��ϵ�д�
 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д� Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д�
�����Ŀ


 ��20mLij���ʵ���Ũ�ȵ�AlCl3��Һ�е���2mol/L NaOH��Һʱ���õ���Al��OH��3�������������μ�NaOH��Һ�������mL����ϵ��ͼ��ʾ���Իش��������⣺
��20mLij���ʵ���Ũ�ȵ�AlCl3��Һ�е���2mol/L NaOH��Һʱ���õ���Al��OH��3�������������μ�NaOH��Һ�������mL����ϵ��ͼ��ʾ���Իش��������⣺