��Ŀ����
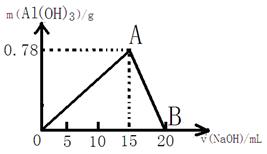
 ��20mLij���ʵ���Ũ�ȵ�AlCl3��Һ�е���2mol/L NaOH��Һʱ���õ���Al��OH��3�������������μ�NaOH��Һ�������mL����ϵ��ͼ��ʾ���Իش��������⣺
��20mLij���ʵ���Ũ�ȵ�AlCl3��Һ�е���2mol/L NaOH��Һʱ���õ���Al��OH��3�������������μ�NaOH��Һ�������mL����ϵ��ͼ��ʾ���Իش��������⣺��1��ͼ��A���ʾ��������
����Al��OH��3���������ֵ
����Al��OH��3���������ֵ
����2��ͼ��B���ʾ��������
Al��OH��3������ȫ�ܽ���NaOH��Һ��ΪNaAlO2��Һ
Al��OH��3������ȫ�ܽ���NaOH��Һ��ΪNaAlO2��Һ
����3��������Ӧ�������ܵ����ӷ���ʽ�ɱ�ʾΪ��
Al3++4OH-=AlO2-+2H2O
Al3++4OH-=AlO2-+2H2O
����4��������Һ����Al��OH��3����0.39g�����ʱ��ȥNaOH��Һ�������
7.5��17.5
7.5��17.5
mL����������1����ʼAlCl3��NaOH��ӦAl3++3OH-=Al��OH��3��������Al��OH��3������NaOH�������࣬Al��OH��3��������A��ʱ�������ƽ�AlCl3ǡ����ȫ����ʱ��Al��OH��3�����ﵽ�������
��2������ټ�NaOH������Al��OH��3+OH-=AlO2-+2H2O�������������٣���B��ʱAl��OH��3��NaOHǡ����ȫ��Ӧ����NaAlO2��������ȫ�ܽ���ʧ��
��3�����ݷ�ӦAl3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+2H2Oд���ܷ�Ӧ��
��4��0.39 g Al��OH��3�����ʵ���Ϊ0.005mol����NaOH��Һ����ʱ������0.39 g Al��OH��3����NaOH�����ʵ���Ϊ0.015 mol����NaOH��Һ����ʱ����ʣ��0.39 g Al��OH��3��ʣ��0.39 g Al��OH��3�ܽ��������0.005molNaOH���ʹ�����NaOH�����ʵ���=0.02L��2mol/L-0.005mol=0.035 mol������V=
����NaOH��Һ�������
��2������ټ�NaOH������Al��OH��3+OH-=AlO2-+2H2O�������������٣���B��ʱAl��OH��3��NaOHǡ����ȫ��Ӧ����NaAlO2��������ȫ�ܽ���ʧ��
��3�����ݷ�ӦAl3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+2H2Oд���ܷ�Ӧ��
��4��0.39 g Al��OH��3�����ʵ���Ϊ0.005mol����NaOH��Һ����ʱ������0.39 g Al��OH��3����NaOH�����ʵ���Ϊ0.015 mol����NaOH��Һ����ʱ����ʣ��0.39 g Al��OH��3��ʣ��0.39 g Al��OH��3�ܽ��������0.005molNaOH���ʹ�����NaOH�����ʵ���=0.02L��2mol/L-0.005mol=0.035 mol������V=
| n |
| c |
����⣺��1����ʼAlCl3��NaOH��ӦAl3++3OH-=Al��OH��3��������Al��OH��3������NaOH�������࣬Al��OH��3��������A��ʱ�������ƽ�AlCl3ǡ����ȫ����ʱ��Al��OH��3�����ﵽ�������
�ʴ�Ϊ������Al��OH��3���������ֵ��
��2��Al��OH��3�����ﵽ��������ټ�NaOH������Al��OH��3+OH-=AlO2-+2H2O�������������٣���B��ʱAl��OH��3��NaOHǡ����ȫ��Ӧ����NaAlO2��������ȫ�ܽ���ʧ��
�ʴ�Ϊ��Al��OH��3������ȫ�ܽ���NaOH��Һ��ΪNaAlO2��Һ��
��3���ɷ�ӦAl3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+2H2O�������������������ӵ��ܷ�ӦΪ��Al3++4OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al3++4OH-=AlO2-+2H2O��
������ԭ���غ㣬��n��AlCl3��=n[Al��OH��3]=0.01mol��
��4��0.39 g Al��OH��3�����ʵ���=
=0.005mol��
��NaOH��Һ����ʱ������0.39 g Al��OH��3����NaOH�����ʵ���Ϊ��0.005mol��3=0.015 mol����ҪNaOH��Һ�����=
=0.0075L=7.5mL��
��NaOH��Һ����ʱ����ʣ��0.39 g Al��OH��3��ʣ��0.39 g Al��OH��3�ܽ��������0.005molNaOH���ʹ�����NaOH�����ʵ���=0.02L��2mol/L-0.005mol=0.035 mol����ҪNaOH��Һ�����=
=0.0175L=17.5mL��
�ʴ�Ϊ��7.5 ��17.5��
�ʴ�Ϊ������Al��OH��3���������ֵ��
��2��Al��OH��3�����ﵽ��������ټ�NaOH������Al��OH��3+OH-=AlO2-+2H2O�������������٣���B��ʱAl��OH��3��NaOHǡ����ȫ��Ӧ����NaAlO2��������ȫ�ܽ���ʧ��
�ʴ�Ϊ��Al��OH��3������ȫ�ܽ���NaOH��Һ��ΪNaAlO2��Һ��
��3���ɷ�ӦAl3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+2H2O�������������������ӵ��ܷ�ӦΪ��Al3++4OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al3++4OH-=AlO2-+2H2O��
������ԭ���غ㣬��n��AlCl3��=n[Al��OH��3]=0.01mol��
��4��0.39 g Al��OH��3�����ʵ���=
| 0.39g |
| 78g/mol |
��NaOH��Һ����ʱ������0.39 g Al��OH��3����NaOH�����ʵ���Ϊ��0.005mol��3=0.015 mol����ҪNaOH��Һ�����=
| 0.015mol |
| 2mol/L |
��NaOH��Һ����ʱ����ʣ��0.39 g Al��OH��3��ʣ��0.39 g Al��OH��3�ܽ��������0.005molNaOH���ʹ�����NaOH�����ʵ���=0.02L��2mol/L-0.005mol=0.035 mol����ҪNaOH��Һ�����=
| 0.035mol |
| 2mol/L |
�ʴ�Ϊ��7.5 ��17.5��
������������AlCl3��NaOH��Ӧ��ͼ�����ϵļ������⣬������ѧ����ͼ��Ľ�������������������������������ȣ��Ѷ��еȣ��������Ĺؼ�����ȷNaOH��Һ��μ��뵽AlCl3��Һ�еķ�Ӧ�����

��ϰ��ϵ�д�
�����Ŀ
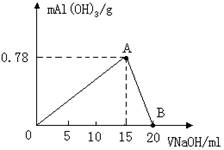
 ��20mLij���ʵ���Ũ�ȵ�AlCl3��Һ�еμ�2mol/L NaOH��Һʱ����μ���NaOH��Һֱ�����������ⶨ�������NaOH��Һ�������mL�������ó��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ����
��20mLij���ʵ���Ũ�ȵ�AlCl3��Һ�еμ�2mol/L NaOH��Һʱ����μ���NaOH��Һֱ�����������ⶨ�������NaOH��Һ�������mL�������ó��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ����