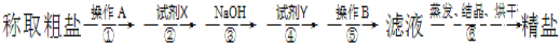��Ŀ����
����Ŀ��ʹ������ƿ������Һʱ�����ڲ��������������������������ʹ������ҺŨ��ƫ�͵��ǣ� ��
������ƽ(ʹ������)����ʱ�����������������λ�÷ŵߵ�
����Һת�Ƶ�����ƿ���ձ���������δ������ˮϴ��
��ת����Һǰ����ƿ������������ˮ
�ܶ���ʱ����������ƿ�Ŀ̶���
�ݶ��ݺ�ҡ�ȣ�����Һ�潵�ͣ��ֲ�������ˮ�����´ﵽ�̶���
A. �٢ڢ�B. �٢ۢ�C. �ۢܢ�D. �ڢۢ�
���𰸡�A
��������
���ù�ʽ![]() ������������
������������
�ٳ���ʱ����Ʒ������ߵ�����ʹ�����������£���Ʒ�����ˣ����ʵ�����mƫ�ͣ�cƫ�ͣ��ٷ������⣻���ձ���������δ������ˮϴ�ӣ�˵���в�������û��ת�Ƶ�����ƿ�У�����ƿ�����ʵ�����mƫ�ͣ�cƫ�ͣ��ڷ������⣻������ƿ������������ˮ���ܽ�֮��IJ�������Ҫ������ƿ�м�ˮ������ԭ������������ˮ���������Ӱ�죬�۲��������⣻�ܶ���ʱ�����ӿ̶ȣ�Һ���ڿ̶������£�VƫС��cƫ�����������⣻�����ݺ��ٲ���ˮ�൱��ϡ�ͣ�cƫС�����������⣻���Ϸ�������Ϊ�٢ڢ��������ѡA��

��ϰ��ϵ�д�
 ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д�
�����Ŀ