��Ŀ����
����Ŀ��Ϊ��ȥ�����е�CaCl2��MgCl2���������Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£�
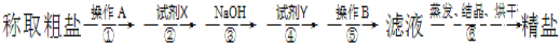
(1)�ڢٲ��У�����A��__________���ڢݲ��У�����B��__________��
(2)�ж��Լ�X�����ķ�����________________________��
(3)д���ڢܲ����漰��Ӧ�Ļ�ѧ����ʽ____________________��
(4)�ڢݲ������еõ��Ĺ���ɷ��У���ɳ��CaCO3��Mg(OH)2��__________���ѧʽ����
(5)��ʵ�鷽���������ƣ����岽����__________________��
���𰸡��ܽ� ���� ȡ�����ϲ���Һ���μ�BaCl2(���Լ�X)��Һ�����ް�ɫ����������˵��BaCl2���� CaCl2+Na2CO3=CaCO3��+2NaCl�� BaCl2+Na2CO3=BaCO3��+2NaCl BaSO4��BaCO3 ����Һ�м���������HCl���к�NaOH����ȥ������Na2CO3
��������
��ȥ�����е�Ca2+��Mg2+��SO42-�Լ���ɳ�����ʣ�������Ϊ�ܽ���м�BaCl2��ȥSO42-�����м�NaOH��ȥMg2+�����м�Na2CO3��ȥCa2+��������BaCl2�����в���BΪ���ˣ����˺���Һ�к���NaOH��Na2CO3��NaCl��ֱ�Ӽ��ȡ������õ��ľ����к�NaOH��Na2CO3��NaCl���Դ˽����⡣
(1)��������������֪���ڢٲ��У�����A���ܽ⣬�ڢݲ��У�����B�ǹ��ˡ�
(2)�ж��Լ�BaCl2�����ķ�����ȡ�����ϲ���Һ���μ�BaCl2(���Լ�X)��Һ�����ް�ɫ����������˵��BaCl2������
(3)�ڢܲ��Ǽ�Na2CO3��ȥCa2+��������BaCl2�������漰��Ӧ�Ļ�ѧ����ʽΪ��CaCl2+Na2CO3=CaCO3��+2NaCl��BaCl2+Na2CO3=BaCO3��+2NaCl��
(4)�ڢݲ������еõ��Ĺ���ɷ��У���ɳ��CaCO3��Mg(OH)2��CaCO3��BaCO3��
(5)��ʵ�鷽����Ϊ��ȥ������MgCl2�����˹�����NaOH��Ϊ��ȥ������CaCl2��������BaCl2�������˹�����Na2CO3���������˳�ȥ���������к����Һ�к�������NaOH��Na2CO3��NaCl��ֱ�������ᾧ��õľ����к�������NaOH��Na2CO3�����ԸĽ��ķ���������Һ�м���������HCl���к�����NaOH������ȥ������Na2CO3��




